


The article examines effective strategies for recruiting healthy volunteers for clinical trials, a critical component in evaluating the safety and tolerability of new medications. It underscores the significance of targeted outreach and clear communication, alongside ethical compliance, to bolster participation rates and safeguard participant well-being. Continuous evaluation of recruitment practices is also highlighted, drawing on statistics and best practices within the field to enhance understanding and implementation.
The foundation of clinical trials is fundamentally anchored in the participation of healthy volunteers, particularly during the pivotal Phase I studies that assess the safety of new medications. Their involvement is crucial, as it establishes essential baselines for comparison and significantly propels the advancement of medical science.
However, the recruitment of these indispensable participants presents unique challenges; many potential volunteers express concerns regarding risks and ethical considerations.
How can researchers effectively engage and reassure healthy volunteers, ensuring the success of their trials while adeptly navigating these complexities?
The success of clinical trials healthy volunteers is essential, particularly in Phase I studies, where the primary objective is to evaluate the safety and tolerability of new medications or interventions. Their involvement establishes a vital baseline for comparison with patient groups, allowing researchers to understand how a drug interacts with a healthy body. This comprehension is crucial for identifying potential side effects and assessing the overall pharmacokinetics of the drug.
Statistics indicate that:
The participation of clinical trials healthy volunteers not only aids in the development of safe medications but also plays a pivotal role in advancing medical science. Their contributions yield invaluable data that can lead to innovative treatments and therapies, ultimately enhancing public health. As the landscape of clinical research evolves in 2025, the significance of clinical trials healthy volunteers remains paramount, ensuring that drug safety and efficacy are rigorously evaluated before reaching broader patient populations. It is also critical to acknowledge that the research is geographically restricted to Sweden, and results should be applied cautiously to other regions. Understanding subjects' motivations and decision-making processes is vital, as emphasized by experts in the field.
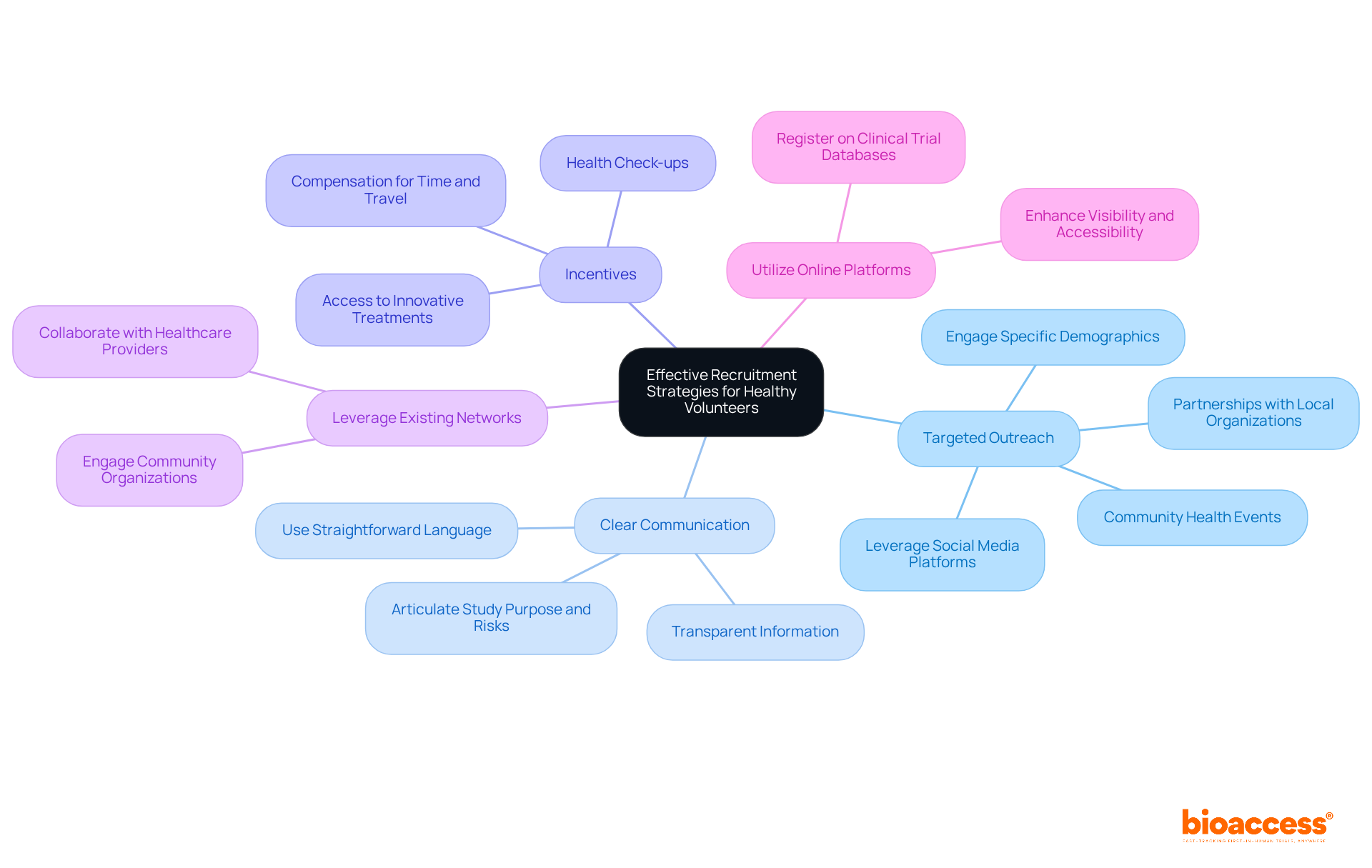
To effectively recruit clinical trials healthy volunteers, it is essential to implement targeted strategies that resonate with your research objectives.
Targeted Outreach: Focus on engaging specific demographics that meet your research criteria. Leverage social media platforms, community health events, and partnerships with local organizations to establish connections with potential volunteers.
Clear Communication: It is crucial to ensure that information regarding the research is transparent and accessible. Clearly articulate the study's purpose, procedures, potential risks, and benefits using straightforward language to minimize confusion.
Incentives: Offering compensation for participants' time and travel, along with additional benefits such as health check-ups or access to innovative treatments, can significantly motivate individuals to participate.
Leverage Existing Networks: Collaborate with healthcare providers and community organizations to enhance outreach efforts. These partners can help identify potential contributors and foster trust within the community, ultimately boosting participation rates.
Utilize online platforms by registering your trial on clinical trial databases that facilitate connections with clinical trials healthy volunteers. This approach improves visibility and accessibility, facilitating easier discovery and engagement for potential participants with your research.
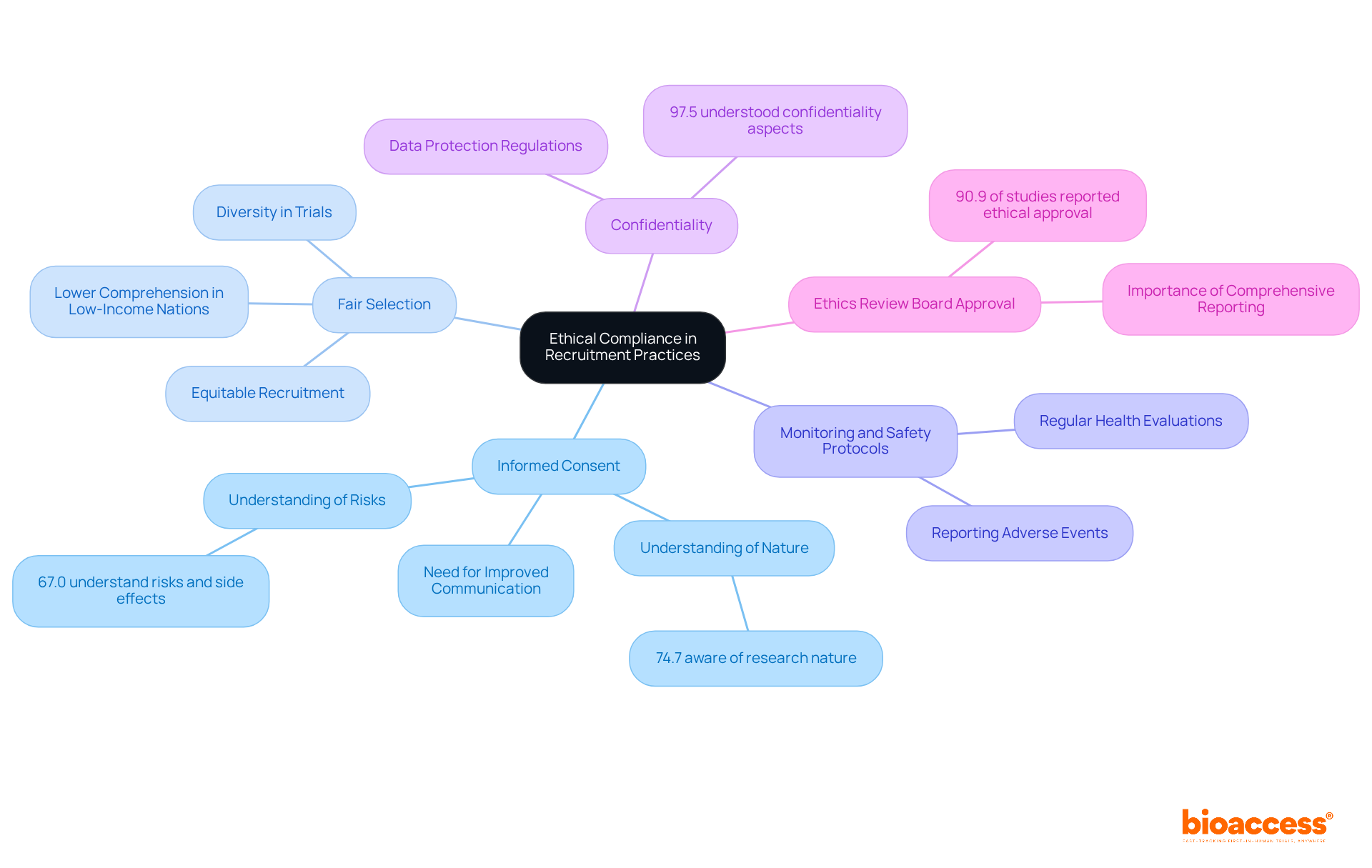
Ensuring ethical compliance in the recruitment of healthy volunteers is paramount in clinical research, involving several key practices:
Informed Consent: Volunteers must be fully informed about the project's nature, risks, and benefits prior to participation. Clear documentation outlining their rights is crucial. Recent findings reveal that comprehension of informed consent elements varies significantly; only 74.7% of individuals are aware of the research's nature, and 67.0% understand potential risks and side effects. This underscores the urgent need for improved communication strategies to enhance comprehension.
Fair Selection: Recruitment must be equitable, avoiding discrimination based on race, gender, or socioeconomic status. A varied group of individuals is essential, including clinical trials healthy volunteers, as research indicates that individuals from low-income nations frequently possess considerably lower comprehension of informed consent elements. Ensuring diversity not only reflects the population but also enriches the data collected.
Monitoring and Safety Protocols: Implementing robust monitoring systems is critical for participant safety throughout the trial. Regular health evaluations and prompt reporting of adverse occurrences are essential to uphold ethical standards and safeguard participants.
Confidentiality: Protecting the personal information of volunteers is paramount. Adhering to data protection regulations ensures that all collected data is stored securely and used solely for the intended research purposes. A significant 97.5% of participants understood confidentiality aspects, highlighting the importance of clear communication in this area.
Ethics Review Board Approval: Before enlisting participants, securing authorization from an ethics review board is crucial to guarantee that the research complies with all ethical norms and directives. Ethical approval was reported in 90.9% of studies, yet comprehensive reporting on informed consent and data handling remains critical to uphold research integrity.
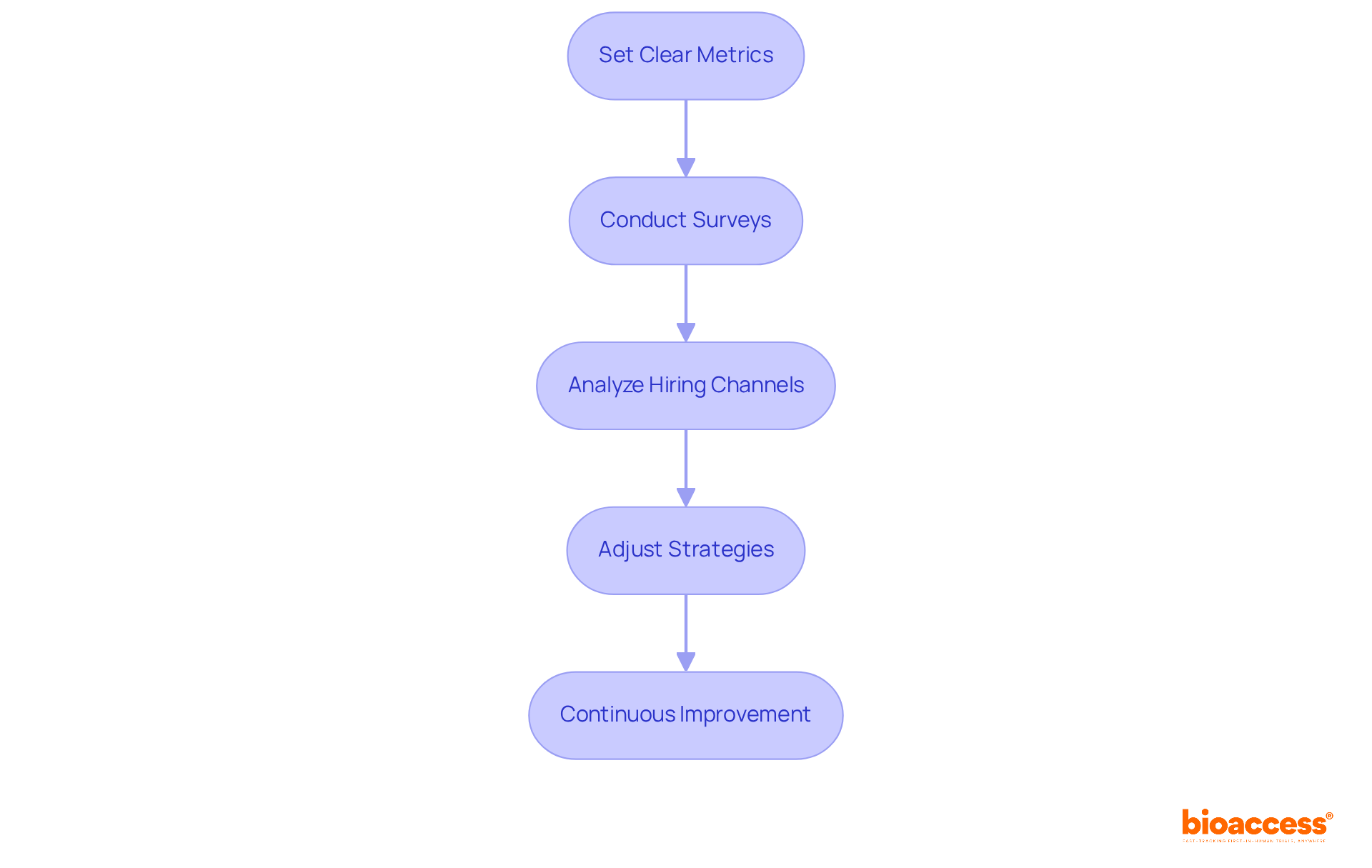
To effectively evaluate and optimize recruitment outcomes in clinical trials, it is essential to follow a structured approach:
Set Clear Metrics: Establish specific measures to evaluate hiring success, including enrollment rates, retention rates, and demographics of attendees. For instance, the median enrollment rate across different studies is approximately 0.92 individuals per center each month. This statistic underscores the importance of monitoring these metrics to identify trends and opportunities for enhancement.
Conduct Surveys: After the trial, implement surveys to gather participant feedback on their enrollment experience. Insights from these surveys can illuminate effective aspects and highlight areas needing improvement, contributing to a more participant-centered approach.
Analyze Hiring Channels: Assess the effectiveness of various avenues in attracting volunteers. Notably, individual-to-individual hiring techniques have shown greater success rates compared to online advertisements. This insight can significantly influence future resource allocation and strategy development.
Adjust Strategies: Based on the evaluation, refine hiring strategies to address identified weaknesses. This may involve modifying messaging, targeting diverse demographics, or enhancing participant incentives. Research indicates that government funding and appropriate compensation positively impact enrollment success.
Continuous Improvement: Establish a feedback loop where lessons learned from each trial inform future hiring strategies. This iterative process is crucial, particularly given that up to 85% of clinical trials fail to meet their recruitment targets. Such statistics emphasize the necessity for ongoing adaptation and optimization of recruitment efforts.
The significance of healthy volunteers in clinical trials is paramount, particularly as the landscape of medical research evolves. Their participation is essential for establishing safety baselines and understanding the effects of new interventions prior to their administration to broader patient populations. By contributing to the foundational knowledge of drug interactions within a healthy body, these volunteers play an indispensable role in advancing medical science and improving public health outcomes.
Key insights from this article underscore effective recruitment strategies, the importance of ethical compliance, and the necessity for continuous evaluation of recruitment practices. Targeted outreach, clear communication, and appropriate incentives are vital for attracting healthy volunteers, while ethical considerations ensure that participants are treated with respect and transparency. Furthermore, establishing metrics for evaluating recruitment success enables researchers to refine their strategies, fostering a more efficient and participant-centered approach.
As the demand for innovative therapies increases, so too does the need for effective recruitment of healthy volunteers. It is imperative for researchers and organizations to prioritize ethical practices and optimize their recruitment strategies to ensure that clinical trials can achieve their objectives. By doing so, they not only enhance the quality of research but also contribute to the ongoing advancement of medical science that ultimately benefits society as a whole. Engaging healthy volunteers transcends mere necessity; it is a vital partnership in the journey toward safer and more effective healthcare solutions.
What is the role of healthy volunteers in clinical trials?
Healthy volunteers play a crucial role in clinical trials, especially in Phase I studies, where their involvement helps evaluate the safety and tolerability of new medications or interventions. They establish a baseline for comparison with patient groups, allowing researchers to understand how a drug interacts with a healthy body.
Why is understanding the interaction of drugs with healthy bodies important?
Understanding how a drug interacts with a healthy body is essential for identifying potential side effects and assessing the overall pharmacokinetics of the drug, which is critical for determining its safety and efficacy.
What are some statistics regarding compensation for healthy volunteers?
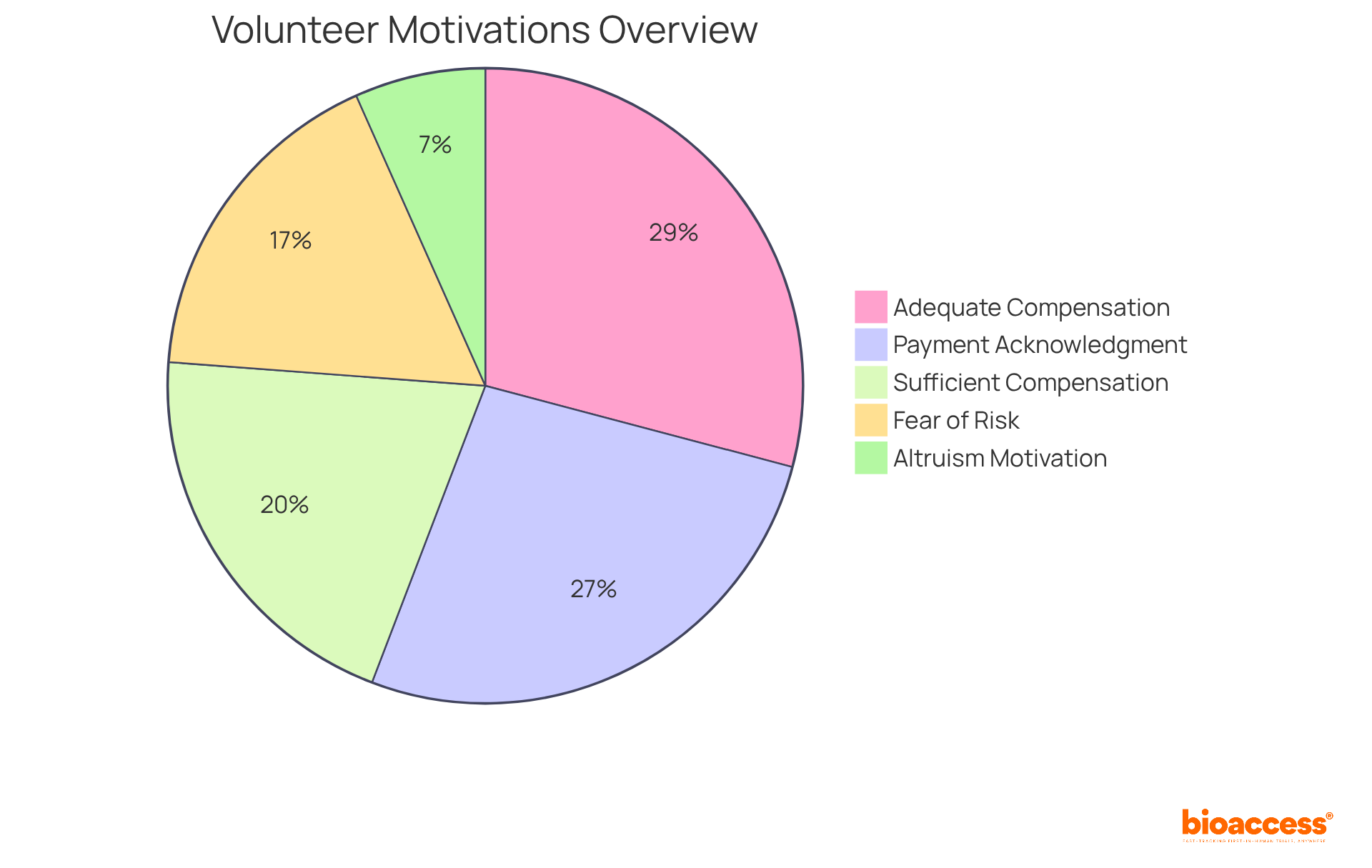
Statistics indicate that 90% of participants find compensation adequate relative to the anticipated risks, 63% deem the compensation amount sufficient, and 82.5% consider payment a necessary acknowledgment of their time and discomfort.
What motivates healthy volunteers to participate in clinical trials?
While financial compensation is a factor, 20.6% of participants report that it is not their sole motivation. Many cite altruism and a desire to contribute to scientific progress as significant reasons for their participation.
What concerns do potential participants have about joining clinical trials?
A notable concern is the fear of risk, with 53% of participants expressing this as the primary reason for not participating in clinical trials.
How do healthy volunteers contribute to medical science?
The participation of healthy volunteers aids in the development of safe medications and provides invaluable data that can lead to innovative treatments and therapies, ultimately enhancing public health.
Are the findings from clinical trials involving healthy volunteers applicable worldwide?
The research discussed is geographically restricted to Sweden, and results should be applied cautiously to other regions due to potential differences in demographics and health profiles.
Why is understanding subjects' motivations important in clinical trials?
Understanding subjects' motivations and decision-making processes is vital for improving recruitment strategies and ensuring that clinical trials are designed in a way that addresses participants' concerns and encourages engagement.