


The article underscores the design and implementation of control treatment groups in clinical trials, highlighting their essential role in assessing the efficacy and safety of new interventions. It articulates that effective control groups empower researchers to isolate treatment effects from external variables, thereby bolstering the validity of study results. This is evidenced by the statistic that approximately 70% of clinical studies employ such groups to uphold rigorous and ethical research practices.
Control treatment groups serve as the backbone of clinical trials, providing essential benchmarks that enable researchers to measure the effectiveness of new interventions. By comparing outcomes against these groups, which may receive placebos or standard care, the integrity and reliability of clinical research are significantly enhanced. However, the design and implementation of these groups present unique challenges that can impact study results.
What strategies can researchers employ to navigate these complexities and ensure robust findings in their clinical trials?
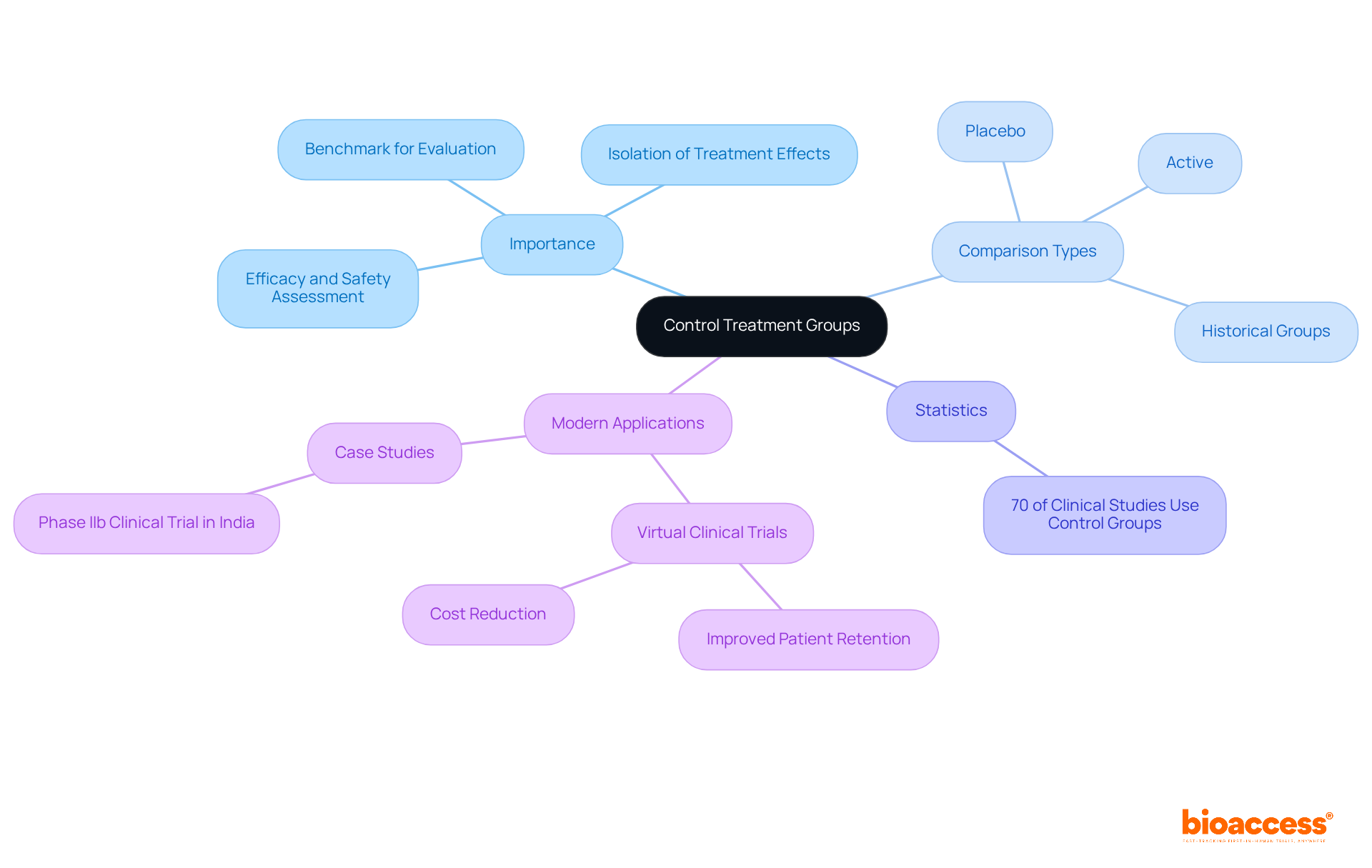
Control treatment groups are essential components of clinical trials, serving as a benchmark against which the effects of experimental interventions can be evaluated. These groups typically consist of individuals in the control treatment group who are receiving a placebo or standard care rather than the new intervention under investigation. The value of these comparison sets, which include the control treatment group, lies in their ability to provide a reference point that helps researchers assess the efficacy and safety of the new treatment. By isolating the treatment's effects from other variables, the control treatment group enhances the reliability of study outcomes and confirms that any observed effects are attributable to the treatment itself, not external factors. Understanding the different types of experimental groups, such as the control treatment group, active, placebo, and historical groups, is crucial for designing robust clinical studies.
Statistics indicate that approximately 70% of clinical studies utilize a control treatment group, underscoring their prevalence and importance in research. The World Medical Association asserts that the benefits, risks, and effectiveness of new methods should be evaluated against the best available therapies, which may necessitate the use of a control treatment group, including a placebo or no intervention, when no proven alternatives exist. This principle highlights the critical role of the control treatment group and comparison samples in clinical research, ensuring that studies are conducted with rigor and integrity.
Recent studies have underscored the effectiveness of comparison sets in the control treatment group across various therapeutic domains, demonstrating their contribution to advancing medical knowledge and treatment options. For instance, a recent Phase IIb clinical trial in India successfully employed a control treatment group to evaluate a new therapeutic approach, yielding valuable insights by comparing outcomes with a standard intervention. Furthermore, the ability of virtual clinical trials to improve patient retention and reduce costs compared to traditional studies is a significant consideration in modern clinical research, showcasing innovative strategies for utilizing reference populations.
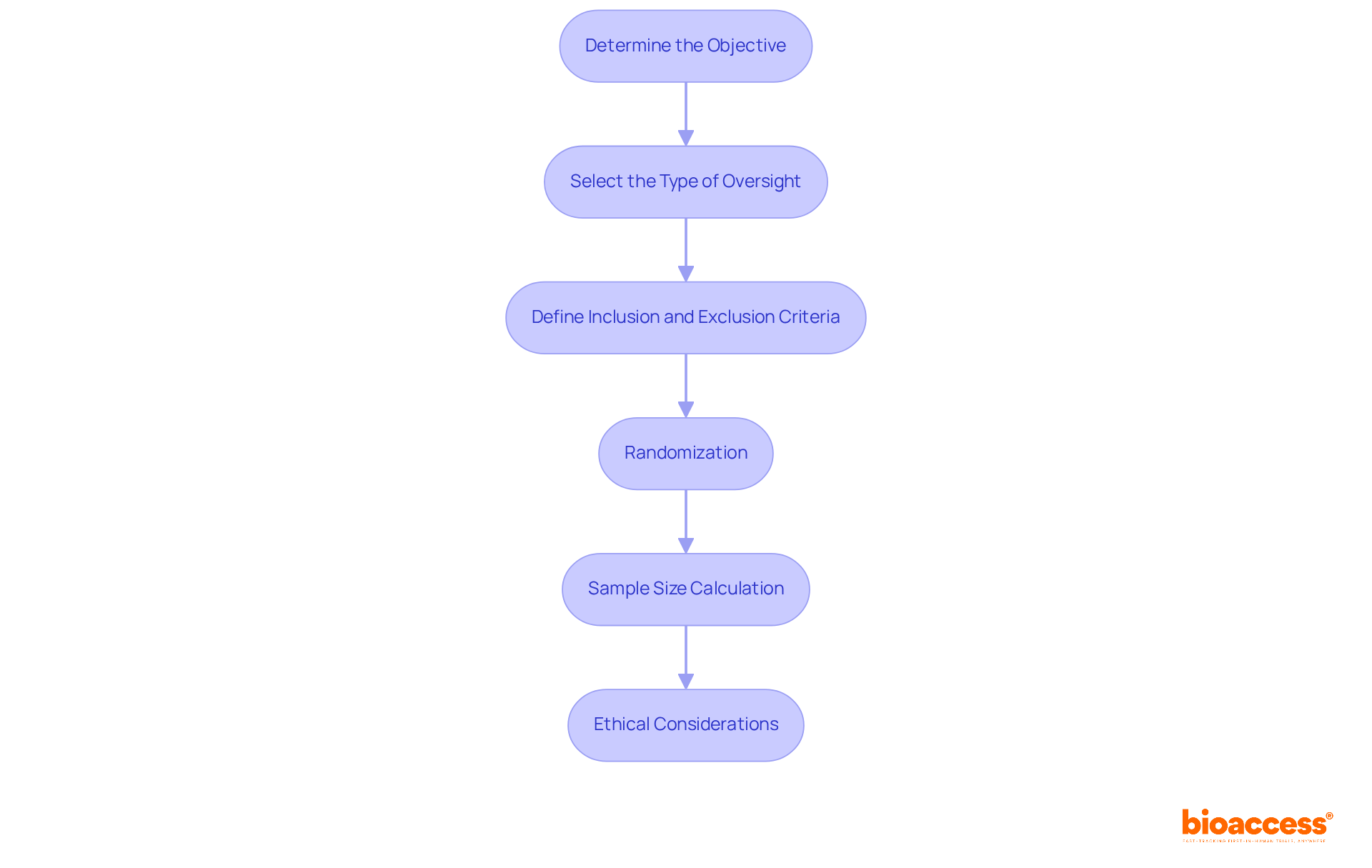
Designing effective control treatment groups is crucial in clinical research, requiring several key steps:
Determine the Objective: Clearly define the primary and secondary aims of the study. This clarity directs the choice of a suitable control treatment group, ensuring it aligns with the research goals.
Select the Type of Oversight: Choose between placebo, active, or historical options based on the trial's objectives and the nature of the intervention. For instance, if testing a new drug, using a control treatment group may be appropriate to establish a clear baseline.
Define Inclusion and Exclusion Criteria: Establish rigorous standards for participant selection to ensure that the comparison cohort mirrors the intervention cohort. This includes demographic factors, health status, and other relevant characteristics that could influence outcomes within the control treatment group.
Randomization: Implement randomization methods to allocate participants to the experimental and treatment categories. This process reduces bias and guarantees comparability between the control treatment group and other groups, enhancing the study's validity.
Sample Size Calculation: Accurately calculate the necessary sample size for the reference cohort to ensure sufficient statistical power. This calculation involves considering the expected effect size and variability in the data for the control treatment group, which are critical for drawing robust conclusions.
Ethical Considerations: Ensure that the design adheres to ethical guidelines, including informed consent and a clear rationale for utilizing a comparison set. Upholding ethical standards is essential for maintaining integrity in the control treatment group of clinical research.
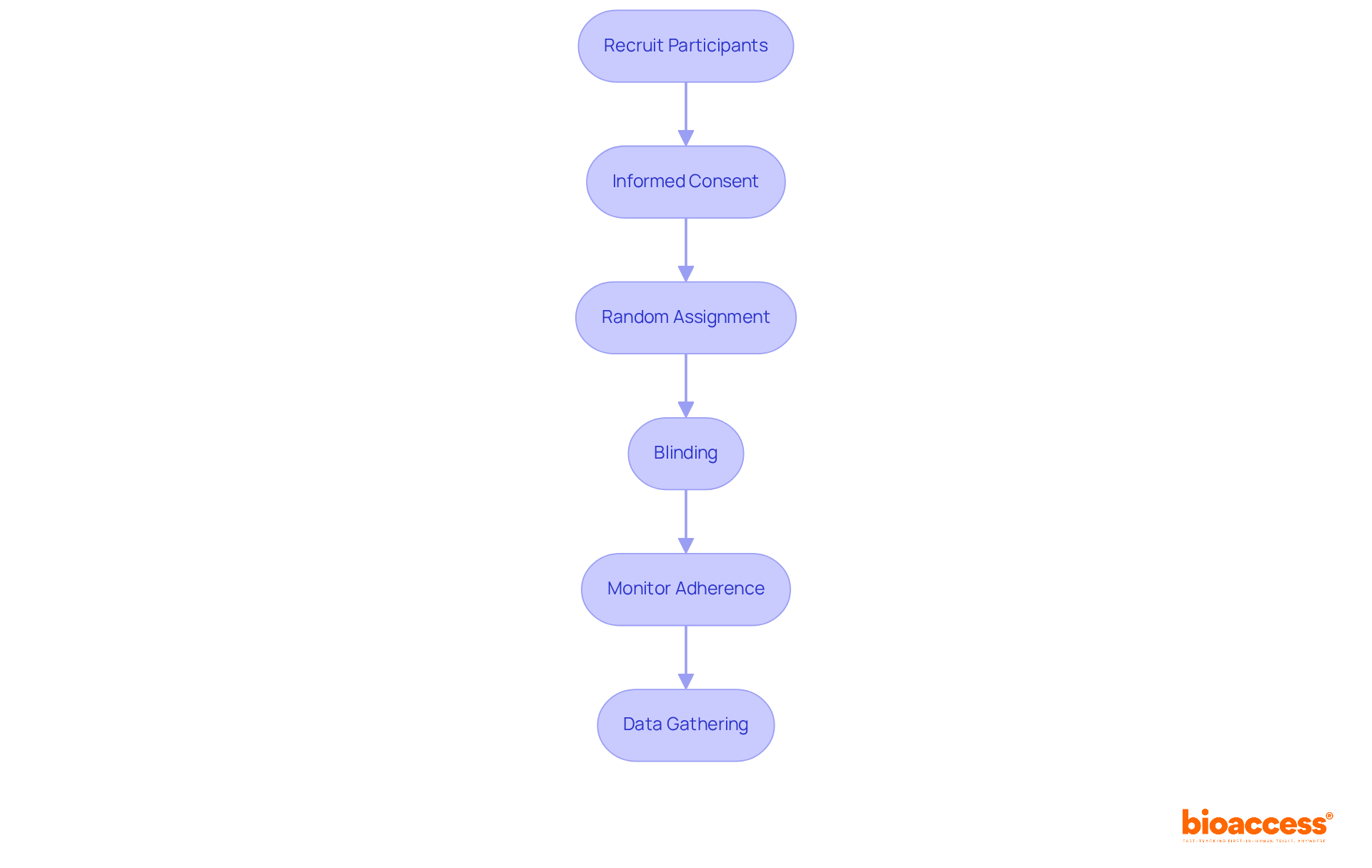
Implementing control treatment groups in clinical trials entails several critical steps that demand attention and precision:
Recruit Participants: Initiate the process by recruiting participants based on the established inclusion and exclusion criteria. It is imperative that the recruitment process remains transparent and ethical.
Informed Consent: Secure informed consent from all participants, clearly delineating the trial's purpose, the function of the comparison group, and any potential risks involved.
Random Assignment: Employ randomization techniques to allocate participants to either the experimental or comparison category. This can be effectively achieved using software or random number tables, ensuring an unbiased allocation.
Blinding: Implement blinding techniques, whether single or double, to mitigate bias. In single-blind studies, participants remain unaware of their assigned category, while in double-blind studies, neither participants nor researchers are informed of the assignments.
Monitor Adherence: Throughout the study, it is crucial to monitor participant adherence to the assigned regimen or comparison group. This may involve regular check-ins or assessments to ensure compliance.
Data Gathering: Methodically gather information from both the reference and experimental groups, ensuring that the data collected is precise and comprehensive for subsequent analysis.
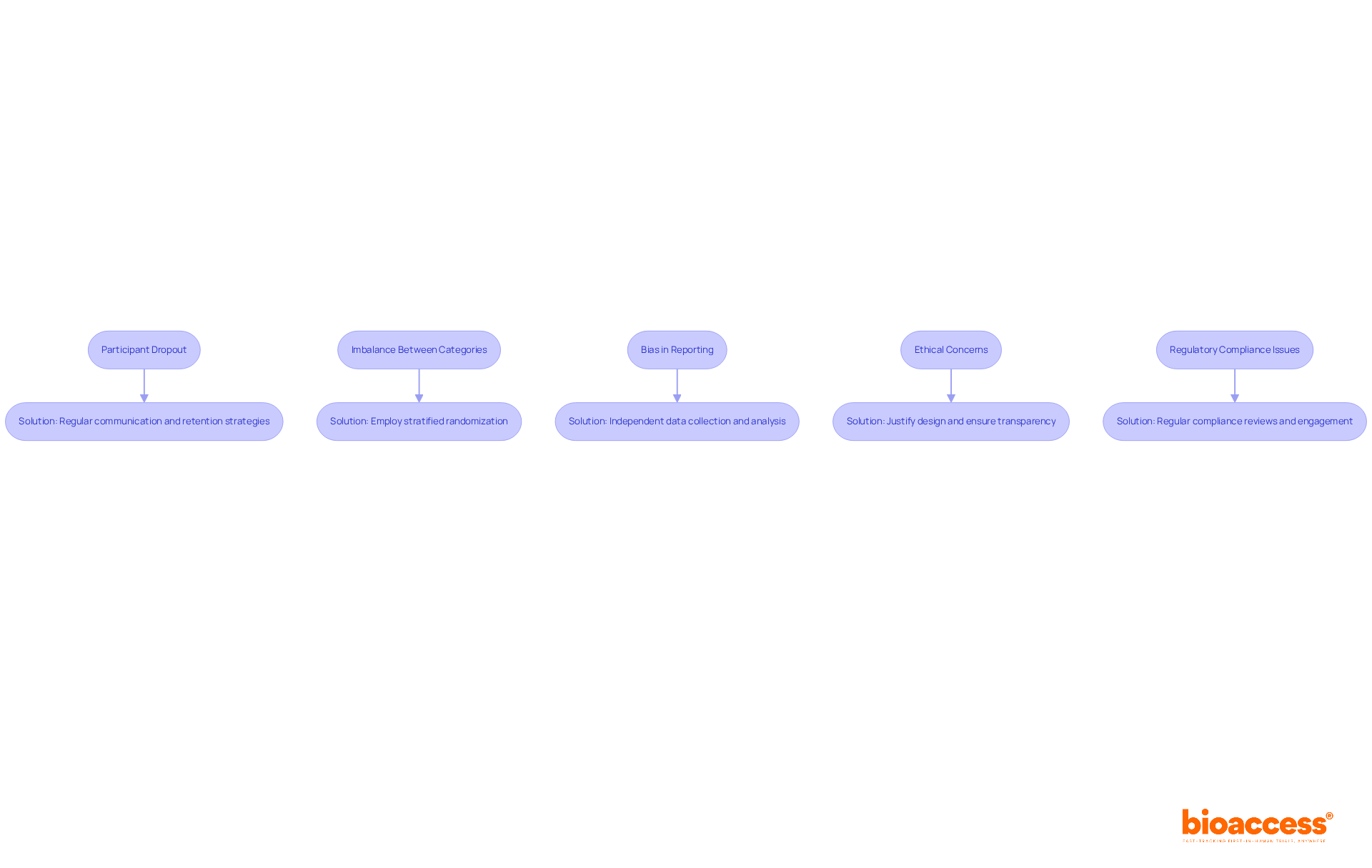
Troubleshooting common issues with the control treatment group is crucial for maintaining the integrity of clinical trials. Below are several prevalent challenges along with effective solutions:
Participant Dropout: When participants withdraw from the study, it can significantly skew results. To mitigate this risk, ensure regular communication and support for participants. Additionally, consider implementing retention strategies such as follow-up calls or incentives to enhance engagement.
Imbalance Between Categories: If randomization fails to yield balanced categories, employing stratified randomization can be an effective solution. This approach ensures that key characteristics are evenly distributed across categories, thereby enhancing the validity of the trial.
Bias in Reporting: To reduce bias in outcome reporting, it is essential that data collection and analysis are conducted by independent parties who remain unaware of the assignments. This practice fosters objectivity and enhances the reliability of the findings.
Ethical concerns: Should ethical concerns arise regarding the use of a control treatment group, be prepared to justify the design thoroughly. It is imperative that participants are fully informed about their involvement, ensuring transparency and ethical compliance.
Regulatory Compliance Issues: Regularly reviewing compliance with regulatory requirements throughout the trial is essential. Engaging with regulatory bodies early in the process allows for proactive identification and resolution of potential issues, safeguarding the trial's integrity.
Control treatment groups are integral to clinical trials, serving as essential benchmarks that empower researchers to assess the efficacy and safety of new interventions. By offering a point of comparison, these groups effectively isolate the treatment effects from other influencing factors, thereby bolstering the reliability and integrity of study outcomes. Mastering the design and implementation of these groups is crucial for conducting rigorous clinical research.
This article has delved into key facets of control treatment groups, highlighting their significance, design considerations, implementation steps, and strategies for troubleshooting common issues. From defining objectives and establishing ethical guidelines to employing randomization and monitoring participant adherence, each step is critical in ensuring that the control treatment group meaningfully contributes to the research findings. The insights presented underscore the necessity of a well-structured approach to clinical trials, particularly in the pursuit of advancing medical knowledge and treatment options.
Given the complexities inherent in designing and implementing control treatment groups, it is essential for researchers to remain vigilant and proactive. By adhering to best practices and confronting potential challenges directly, the integrity of clinical trials can be preserved, ultimately resulting in more reliable outcomes that benefit the broader medical community. Embracing these principles not only elevates the quality of research but also cultivates trust in the findings that will shape future healthcare innovations.
What are control treatment groups in clinical trials?
Control treatment groups are essential components of clinical trials that serve as a benchmark against which the effects of experimental interventions can be evaluated. They typically consist of individuals receiving a placebo or standard care instead of the new intervention being investigated.
Why are control treatment groups important?
Control treatment groups provide a reference point that helps researchers assess the efficacy and safety of new treatments. They isolate the treatment's effects from other variables, enhancing the reliability of study outcomes and confirming that any observed effects are due to the treatment itself.
What types of experimental groups are there in clinical studies?
In addition to control treatment groups, there are several types of experimental groups, including active groups, placebo groups, and historical groups. Understanding these different types is crucial for designing robust clinical studies.
How common are control treatment groups in clinical research?
Approximately 70% of clinical studies utilize a control treatment group, highlighting their prevalence and importance in research.
What does the World Medical Association say about control treatment groups?
The World Medical Association asserts that the benefits, risks, and effectiveness of new methods should be evaluated against the best available therapies, which may require the use of a control treatment group, including a placebo or no intervention, when no proven alternatives exist.
How have recent studies demonstrated the effectiveness of control treatment groups?
Recent studies have shown that control treatment groups contribute to advancing medical knowledge and treatment options across various therapeutic domains. For example, a Phase IIb clinical trial in India successfully used a control treatment group to evaluate a new therapeutic approach, providing valuable insights by comparing outcomes with a standard intervention.
What are the advantages of virtual clinical trials regarding control treatment groups?
Virtual clinical trials can improve patient retention and reduce costs compared to traditional studies, showcasing innovative strategies for utilizing reference populations, including control treatment groups.