


This article highlights the essential practices for mastering the Case Report Form (CRF) format, a critical component for success in clinical research. Effective CRF design and management are paramount; clarity, logical organization, and strict adherence to regulatory standards play a vital role in maintaining data integrity. These elements not only enhance the efficiency of clinical trials but also lead to improved data accuracy and faster patient enrollment.
In the ever-evolving Medtech landscape, understanding the significance of these practices is crucial. As clinical research faces numerous challenges, the role of well-designed CRFs becomes increasingly important. By focusing on these best practices, researchers can navigate complexities more effectively, ensuring that their studies yield reliable and actionable results.
In conclusion, collaboration among stakeholders is essential to implement these strategies successfully. By prioritizing effective CRF design, the clinical research community can enhance overall trial performance and patient outcomes.
In the complex realm of clinical research, Case Report Forms (CRFs) stand as the essential framework for data collection, ensuring that every detail is captured with utmost precision and clarity. The effectiveness of CRFs, especially in their electronic format, significantly impacts the quality and integrity of clinical trials. They offer notable advantages, including reduced error rates and faster patient enrollment. Yet, as the landscape of modern research continues to evolve, a pressing question arises: how can researchers design and implement CRFs that not only comply with regulatory standards but also enhance the overall efficiency and accuracy of data management?
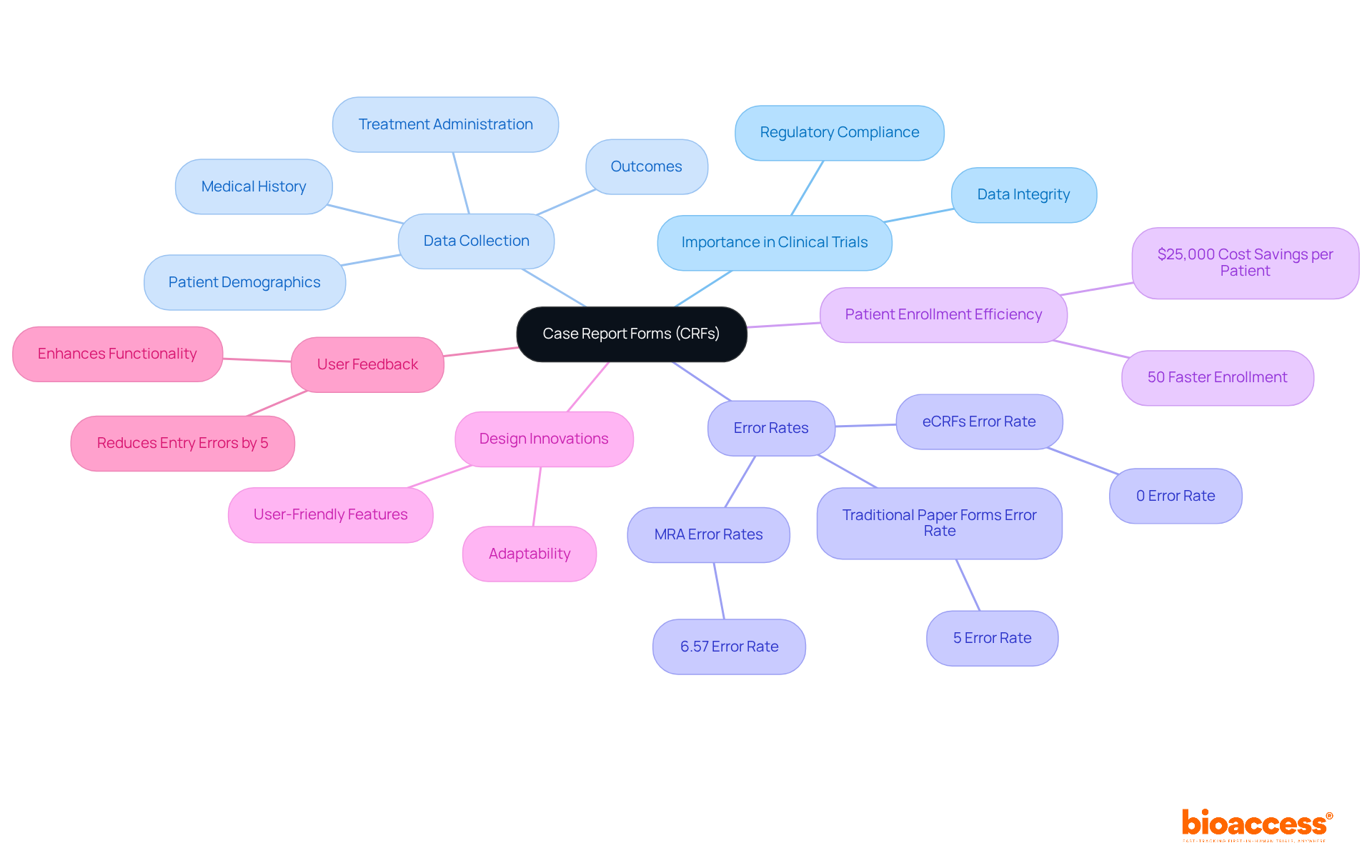
Case Report Forms (CRFs) are essential standardized documents in research studies, meticulously designed to gather information from each participant in a crf format. They capture critical data, including patient demographics, medical history, treatment administration, and outcomes. The significance of CRFs in clinical studies, particularly in crf format, cannot be overstated; they ensure consistent and precise information collection, which is vital for maintaining the integrity of research outcomes. Notably, research shows that electronic CRFs (eCRFs) achieve an impressive 0% error rate, starkly contrasting with the 5% error rate linked to traditional paper forms. This striking difference underscores the effectiveness of eCRFs in managing information quality.
Moreover, the crf format plays a pivotal role in regulatory compliance by providing a clear record of the information collected and the methodologies employed during the study. A well-structured crf format not only enhances the efficiency of data collection but also simplifies analysis, leading to quicker and more reliable study outcomes. For example, studies utilizing bioaccess® have shown that effective CRF design can expedite patient enrollment by 50% compared to conventional methods, resulting in substantial cost savings of $25,000 per patient. This acceleration in patient enrollment not only boosts the study's efficiency but also positively impacts local economies through job creation and healthcare advancements.
In addition to their role in data collection, bioaccess® offers comprehensive services such as feasibility studies and compliance assessments, further streamlining the research process. Recent innovations in the CRF format design emphasize clarity, logical organization, and adaptability, which are crucial for navigating the complexities of modern trials. Incorporating user feedback during the design phase has proven to enhance functionality, further decreasing entry errors by up to 5%. As clinical research protocols evolve, the need for robust CRFs in crf format to address quality challenges becomes increasingly evident, reinforcing their vital role in ensuring data validity and integrity in clinical research.
To ensure quality and compliance in CRF design, several key principles must be implemented:
Clarity and Simplicity: Clear language and straightforward instructions are essential to minimize confusion for entry personnel. Avoiding jargon and ensuring that all terms are well-defined is crucial. As Patricio Ledesma notes, 'To ensure clarity and accuracy, questions in the crf format should be explicit and avoid ambiguity.'
Logical Flow: Organizing the CRF format in a logical sequence that mirrors the patient journey through the trial helps preserve consistency and reduces the chances of missing information, thereby improving overall integrity.
Incorporating built-in validation checks in the crf format is vital for catching errors at the point of information entry. This includes range checks, mandatory fields, and crf format checks to ensure information integrity. Research indicates that user-friendly CRFs significantly reduce entry mistakes, reinforcing the necessity for these checks.
Regulatory Compliance: It is imperative that the crf format adheres to all relevant regulatory guidelines, such as Good Clinical Practice (GCP) and local regulations. This encompasses proper documentation of informed consent and ethical considerations, which are critical for maintaining the integrity of clinical trials.
Conducting pilot testing of the crf format with actual users helps identify potential issues before full-scale implementation. Feedback from users can provide valuable insights for improvement, ensuring that the CRF is both effective and compliant.
Common pitfalls in CRF format design, such as neglecting user-friendliness and consistency, highlight the importance of being aware of these frequent traps. These oversights can lead to increased queries and discrepancies in information. Addressing these challenges through thoughtful planning and teamwork can significantly enhance information quality.
Integrating mechanisms for CRF connectivity and version management in the CRF format is crucial for upholding information integrity across multiple pages and versions. This is essential for accurately monitoring information and preventing the use of outdated or incorrect CRF pages.
By adhering to these design principles, researchers can create CRFs that not only meet regulatory standards but also facilitate effective information gathering, ultimately contributing to the success of clinical studies.

To effectively manage and implement Case Report Forms (CRFs) in clinical trials, adopting best practices for the CRF format is essential.
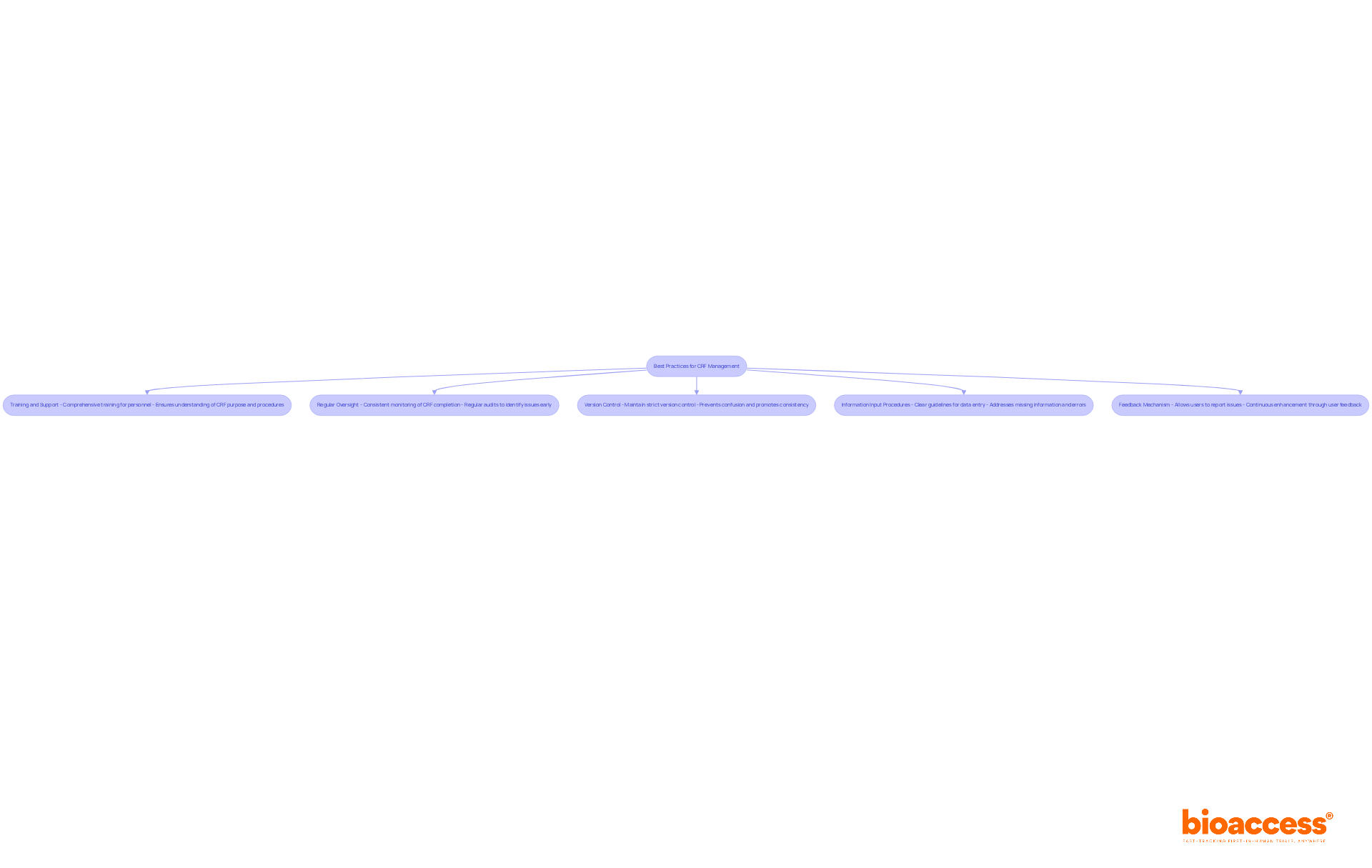
Training and Support: Comprehensive training for all personnel involved in information collection and management is crucial. This guarantees that team members fully understand the purpose, design, and entry procedures of the CRF format, which is vital for preserving information integrity. As noted by CCRPS, "GCP training ensures that trials adhere to ethical standards, respect patient autonomy, and comply with legal obligations such as information protection and patient privacy."
Regular Oversight: Establishing a system for consistent monitoring of CRF completion and information quality is key. Regular audits and evaluations conducted in CRF format help identify inconsistencies or issues early, enabling swift resolution and maintaining high information standards. Statistics indicate that every clinical trial requires one completed CRF for each participant, highlighting the extent of information gathering involved.
Maintaining strict version control of the CRF format is necessary to ensure that all team members are using the most current version. This practice prevents confusion and promotes consistency in information gathering throughout the study, especially when using CRF format.
Implementing a feedback mechanism allows users to report issues or suggest improvements regarding the CRF format. Continuous enhancement driven by user feedback can significantly improve the efficiency of information gathering processes. As highlighted in the case study on "Exploring Electronic Data Capture Applications," technology can play a crucial role in enhancing CRF management and data quality.
By applying these best practices, clinical research teams can enhance the efficiency and accuracy of CRF management, ultimately contributing to the success of clinical trials.
Mastering the Case Report Form (CRF) format is essential for the success of clinical research. Well-designed CRFs not only facilitate accurate data collection but also enhance regulatory compliance and operational efficiency. By adopting best practices in CRF design and management, researchers can mitigate errors, streamline processes, and ultimately contribute to more reliable study outcomes.
Key insights discussed include:
Moreover, electronic CRFs (eCRFs) significantly reduce error rates and improve data quality, showcasing their pivotal role in modern clinical trials.
In summary, the significance of CRFs in clinical research cannot be overstated. As the landscape of clinical trials continues to evolve, prioritizing effective CRF design and management practices is crucial for maintaining data integrity and achieving research goals. Embracing these best practices not only enhances the quality of clinical studies but also fosters innovation and progress in healthcare, ultimately benefiting patients and the broader community.
What are Case Report Forms (CRFs)?
Case Report Forms (CRFs) are standardized documents used in research studies to gather information from each participant, including patient demographics, medical history, treatment administration, and outcomes.
Why are CRFs important in clinical trials?
CRFs are crucial for ensuring consistent and precise information collection, which is vital for maintaining the integrity of research outcomes. They also play a key role in regulatory compliance by providing a clear record of collected information and methodologies.
How do electronic CRFs (eCRFs) compare to traditional paper forms?
Electronic CRFs (eCRFs) achieve a 0% error rate, while traditional paper forms have a 5% error rate, highlighting the effectiveness of eCRFs in managing information quality.
What impact do CRFs have on patient enrollment in clinical trials?
Effective CRF design can expedite patient enrollment by 50% compared to conventional methods, leading to significant cost savings of $25,000 per patient.
How do CRFs contribute to the efficiency of clinical studies?
A well-structured CRF format enhances data collection efficiency and simplifies analysis, resulting in quicker and more reliable study outcomes.
What services does bioaccess® provide related to CRFs?
Bioaccess® offers comprehensive services such as feasibility studies and compliance assessments, which help streamline the research process in addition to CRF design.
What recent innovations have been made in CRF design?
Recent innovations emphasize clarity, logical organization, and adaptability, incorporating user feedback to enhance functionality and decrease entry errors by up to 5%.
Why is the need for robust CRFs becoming increasingly evident?
As clinical research protocols evolve, the need for robust CRFs to address quality challenges becomes more apparent, reinforcing their vital role in ensuring data validity and integrity in clinical research.