


In the realm of clinical trials, data analytics has become indispensable for achieving successful outcomes. This transformation is reshaping how researchers design studies and recruit participants. By leveraging data, research teams can uncover vital insights that not only enhance decision-making but also streamline processes, ultimately leading to more efficient trials. Yet, as data complexity escalates, so do the challenges tied to its analysis. This raises a critical question: how can research teams effectively navigate these obstacles to ensure compliance, uphold ethical standards, and harness advanced technologies for optimal results?
Data analysis clinical trials plays a pivotal role in empowering researchers to derive actionable insights from extensive datasets. Through data analysis clinical trials, identifying patterns and predicting outcomes not only refines study designs but also enhances decision-making processes. For example, leveraging historical data aids in selecting appropriate endpoints and participant groups, leading to more effective studies. Furthermore, analytics significantly boosts recruitment strategies by pinpointing suitable candidates based on specific criteria, which in turn increases enrollment rates and reduces timelines. Understanding these dynamics enables research teams to make informed, data-driven decisions through data analysis clinical trials, which elevate the chances of success.
Real-world applications of predictive analytics, such as forecasting participant dropout rates, facilitate proactive strategies to retain individuals and maintain study integrity. This approach not only enhances efficiency in experiments but also contributes to higher success rates, underscoring the critical importance of data analysis clinical trials in the research landscape. With bioaccess®'s FDA-ready data, research teams can enroll treatment-naive cardiology or neurology groups 50% faster than traditional Western sites, achieving remarkable savings of $25K per patient. This capability addresses common hiring challenges faced by Medtech and biopharma startups, allowing them to navigate the complexities of early-stage research more effectively. Moreover, the financial implications of enrollment delays are significant, with approximately 80% of studies failing to meet initial enrollment targets, resulting in considerable losses for drug discovery firms. These insights illustrate the tangible impact of strong analytics in the research environment, further supported by bioaccess®'s collaboration with Caribbean Health Group to position Barranquilla as a leading hub for research studies in Latin America.

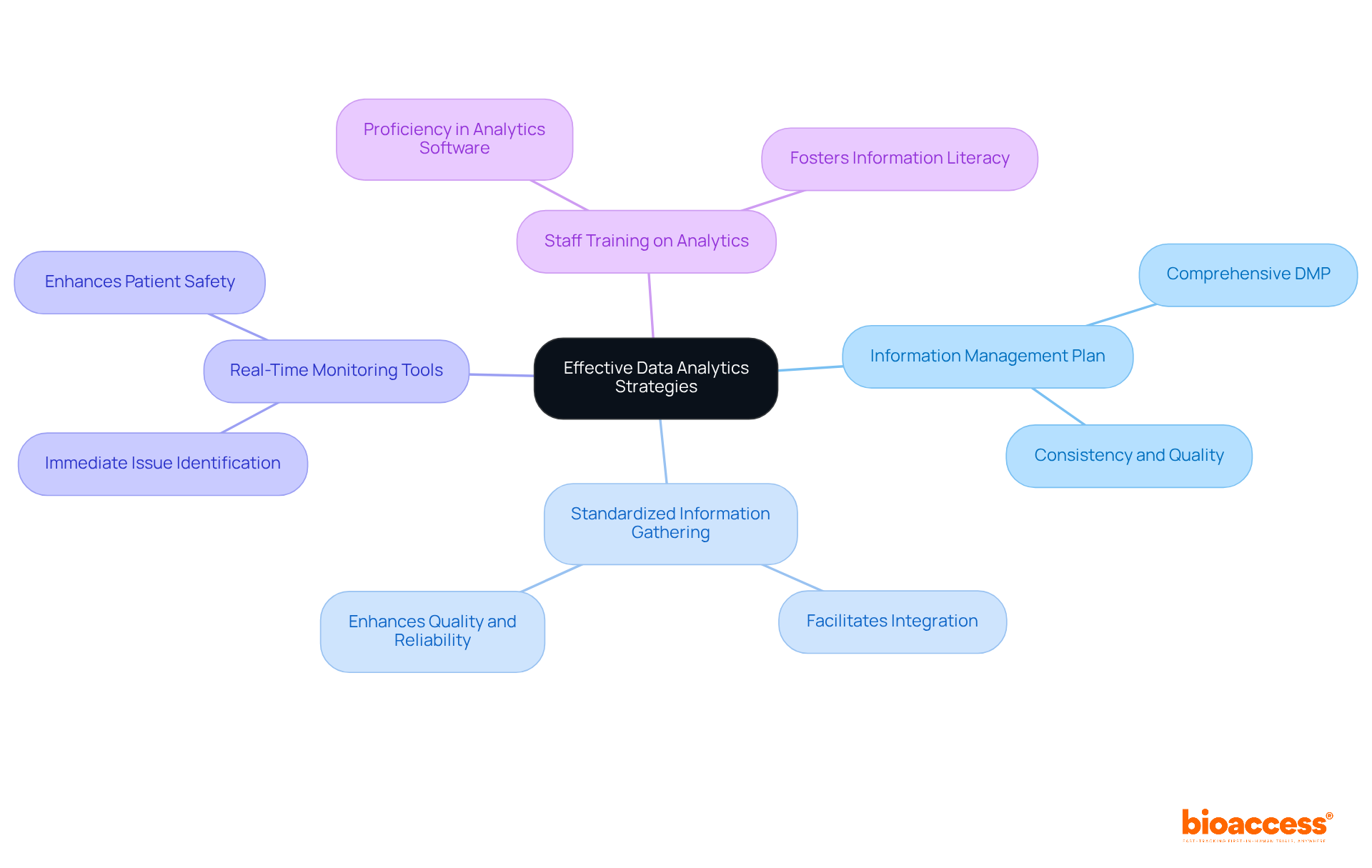
To implement effective data analytics strategies in clinical trials, organizations must prioritize the following best practices:
By embracing these approaches, research teams can significantly enhance their analytics capabilities, leading to better outcomes and more efficient procedures. The integration of real-world information (RWI) and advanced analytics can further refine protocol design, ensuring that studies are not only compliant but also aligned with patient needs and preferences.
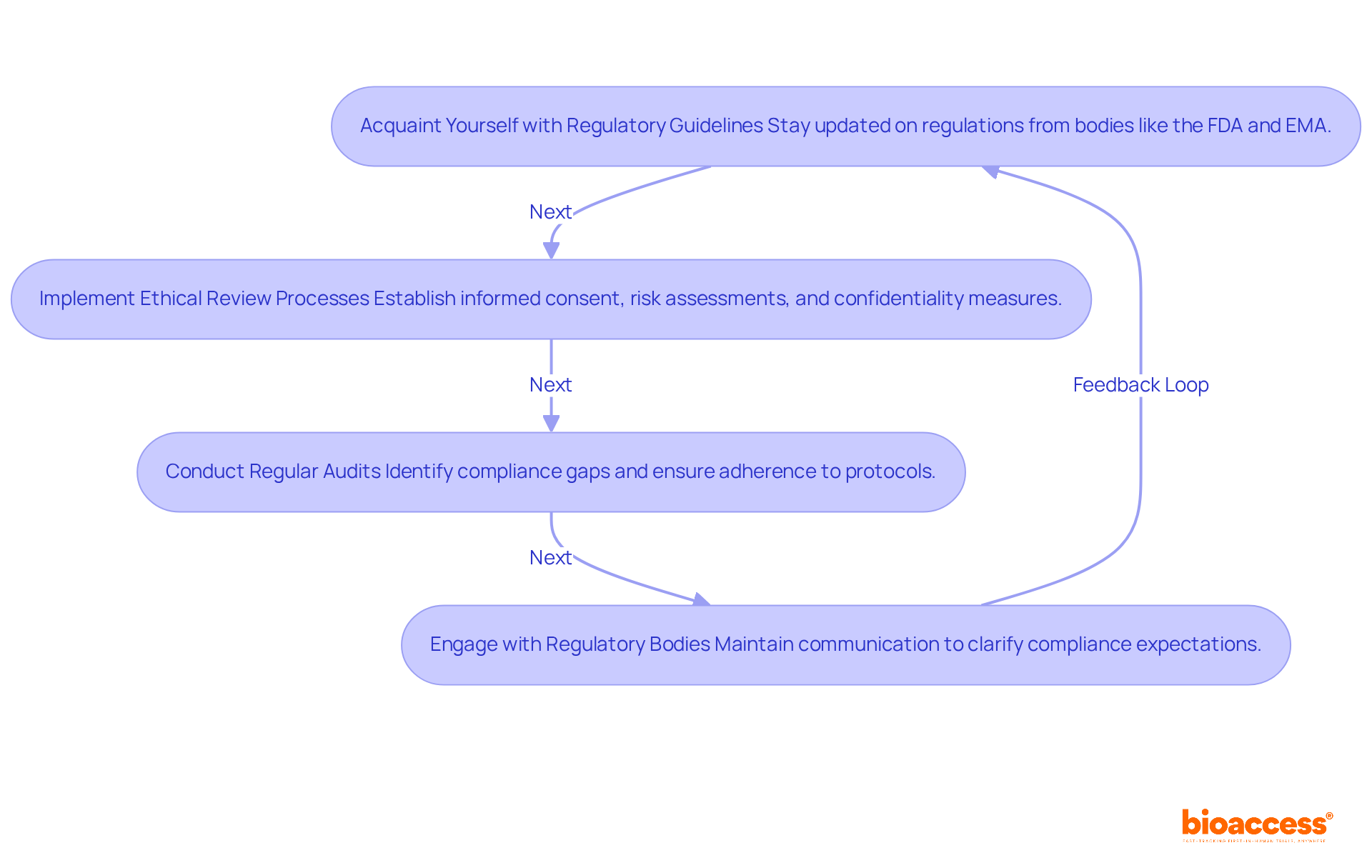
Ensuring adherence to regulatory and ethical standards in research studies is crucial for preserving the integrity of investigations and safeguarding participant rights. Key practices include:
Acquaint Yourself with Regulatory Guidelines: Staying updated on regulations from bodies like the FDA and EMA is essential. These organizations govern the execution of clinical studies and data management protocols, ensuring that research is conducted within established legal frameworks.
Implement Ethical Review Processes: Establishing a comprehensive ethical review process is vital. This should encompass informed consent, thorough risk assessments, and robust measures to protect participant confidentiality, thereby fostering trust and transparency in research.
Conduct Regular Audits: Regular internal audits are indispensable for identifying compliance gaps and ensuring adherence to established protocols. This proactive approach not only reduces risks linked to non-compliance but also enhances the overall integrity of the research process.
Engage with Regulatory Bodies: Maintaining open lines of communication with regulatory authorities clarifies compliance expectations and facilitates smoother research operations. This engagement is critical for navigating the complexities of regulatory landscapes.
By prioritizing these compliance practices, research teams not only uphold ethical standards but also enhance the credibility and reliability of their findings.
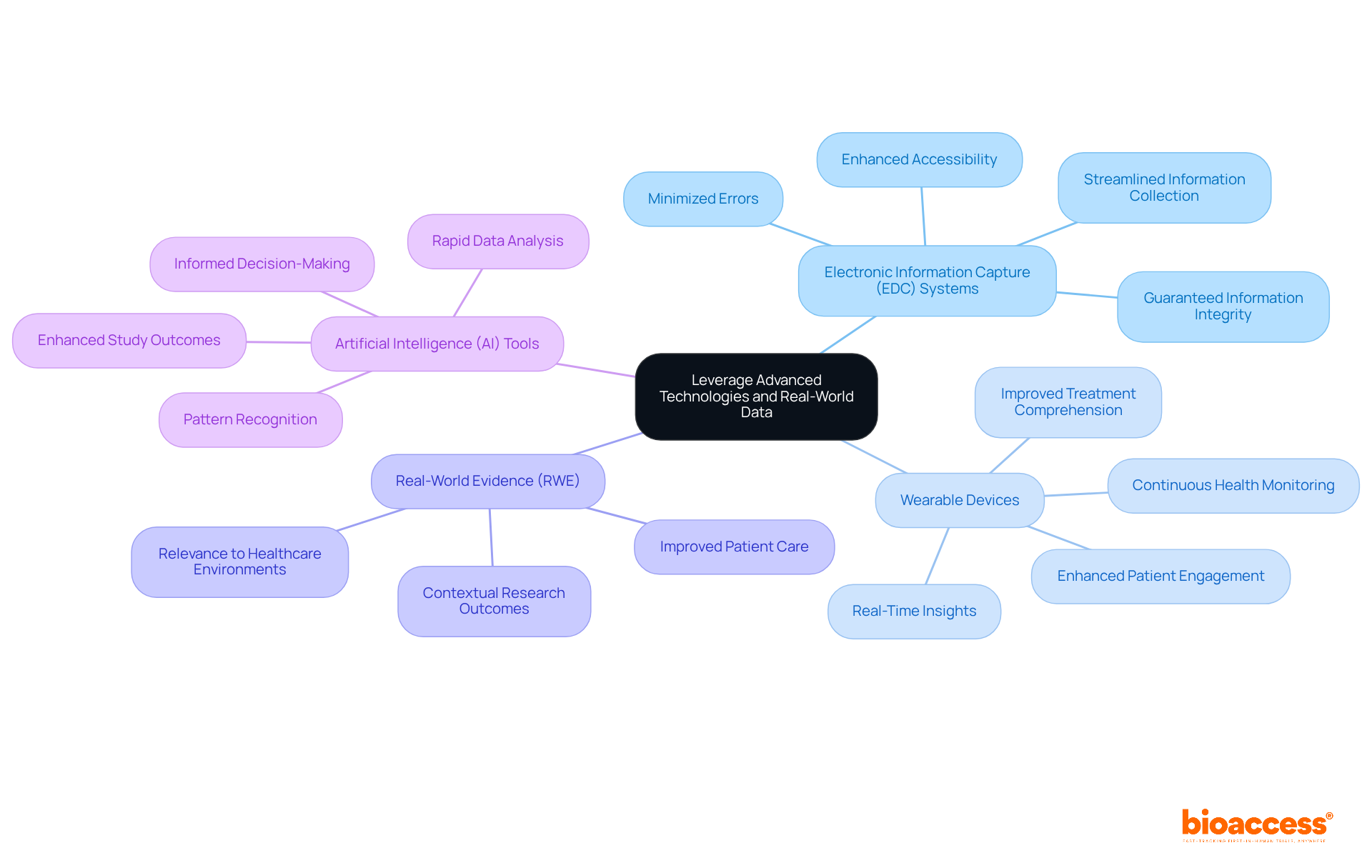
To effectively leverage advanced technologies and real-world data in clinical trials, consider the following strategies:
Adopt Electronic Information Capture (EDC) Systems: EDC systems significantly streamline information collection and management processes, minimizing errors associated with manual entry and enhancing accessibility. This change not only enhances efficiency but also guarantees information integrity throughout the experiment.
Utilize Wearable Devices: Wearable technology enables continuous health monitoring, providing real-time insights into individual conditions. This ability enhances the information gathered during experiments, enabling a more thorough comprehension of treatment impacts and individual responses.
Incorporate Real-World Evidence (RWE): Integrating RWE into study design enhances the relevance of research outcomes by providing context on various individuals and their treatment experiences. This approach results in discoveries that are more relevant to real-world healthcare environments, ultimately enhancing care for individuals.
Implement Artificial Intelligence (AI) Tools: AI tools can rapidly analyze large datasets, uncovering patterns and insights that traditional analysis methods might overlook. By utilizing AI, research teams can improve their analytical abilities, resulting in more informed decision-making and better study outcomes.
Additionally, bioaccess® offers an accelerated regulatory approval process, achieving approvals in just 6-8 weeks compared to the typical 6-12 months in the US/EU. This efficiency enables quicker enrollment of treatment-naive cardiology or neurology groups, overcoming typical recruitment challenges encountered by Medtech and biopharma startups. By integrating these technologies and leveraging bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, clinical trial teams can enhance patient engagement, improve data quality, and drive more successful outcomes through data analysis clinical trials.
Data analytics stands as a cornerstone in clinical trials, transforming vast datasets into actionable insights that drive research success. By harnessing the power of data analysis, researchers refine study designs, enhance decision-making, and ultimately improve patient outcomes. The integration of advanced technologies and real-world data streamlines processes and aligns research with the actual needs of patients, ensuring clinical trials remain relevant and effective.
Throughout this article, we’ve highlighted key strategies for effective data analytics in clinical trials. Establishing a comprehensive information management plan, standardizing data collection, utilizing real-time monitoring tools, and training staff on analytics tools are essential practices that enhance the overall quality and reliability of research. Moreover, compliance with regulatory and ethical standards is paramount, upholding the integrity of studies and protecting participant rights. The incorporation of advanced technologies, such as electronic data capture systems and artificial intelligence, amplifies the potential for success in clinical trials.
As the landscape of clinical research evolves, embracing these best practices in data analysis becomes crucial. Organizations must invest in analytics capabilities and foster a culture of data-driven decision-making. By doing so, research teams can navigate the complexities of clinical trials more effectively, leading to better outcomes and advancements in healthcare. The future of clinical trials hinges on leveraging data analytics, making it imperative for stakeholders to prioritize these strategies for sustained success.
What is the role of data analytics in clinical trials?
Data analytics plays a crucial role in clinical trials by enabling researchers to derive actionable insights from extensive datasets, identifying patterns, and predicting outcomes to refine study designs and enhance decision-making processes.
How does data analysis improve study designs in clinical trials?
By leveraging historical data, data analysis helps in selecting appropriate endpoints and participant groups, leading to more effective studies.
In what ways does analytics enhance recruitment strategies for clinical trials?
Analytics boosts recruitment strategies by pinpointing suitable candidates based on specific criteria, which increases enrollment rates and reduces timelines.
What are real-world applications of predictive analytics in clinical trials?
Predictive analytics can forecast participant dropout rates, allowing for proactive strategies to retain individuals and maintain study integrity, which enhances efficiency and contributes to higher success rates.
How does bioaccess®'s FDA-ready data impact enrollment in clinical trials?
Bioaccess®'s FDA-ready data enables research teams to enroll treatment-naive cardiology or neurology groups 50% faster than traditional Western sites, achieving significant savings of $25K per patient.
What challenges do Medtech and biopharma startups face in early-stage research?
Medtech and biopharma startups often face hiring challenges that complicate early-stage research, which can be navigated more effectively with strong analytics.
What are the financial implications of enrollment delays in clinical trials?
Approximately 80% of studies fail to meet initial enrollment targets, leading to considerable losses for drug discovery firms due to enrollment delays.
How does bioaccess® contribute to the research landscape in Latin America?
Bioaccess® collaborates with Caribbean Health Group to position Barranquilla as a leading hub for research studies in Latin America, further enhancing the importance of strong analytics in the research environment.