


Mastering data integrity in the pharmaceutical industry is crucial for ensuring the reliability of clinical research outcomes. This involves implementing best practices such as:
These practices not only ensure compliance with regulatory standards but also foster a culture of accountability. By doing so, organizations can significantly enhance the trustworthiness of their clinical research findings.
Ensuring data integrity is a critical concern in the pharmaceutical industry, where the accuracy and reliability of information can significantly impact research outcomes and regulatory compliance. This article delves into best practices that organizations can adopt to enhance data integrity, from implementing robust standard operating procedures to investing in comprehensive staff training.
However, as the landscape of regulations evolves, how can companies effectively navigate the complexities of data management while safeguarding the integrity of their clinical research? The answers to these questions are not only vital for compliance but also essential for fostering trust and credibility in clinical outcomes.
Ensuring data integrity in pharma company is paramount, as it encompasses the precision, consistency, and dependability of information throughout its lifecycle. The core principles are as follows:
By grasping these principles, organizations can more effectively navigate the complexities of information management and uphold data integrity in pharma company research outcomes.

To ensure data integrity and compliance, organizations must adopt best practices that are essential for effective clinical research.
Standard Operating Procedures (SOPs): Establish and maintain comprehensive SOPs that clearly outline information handling processes. This guarantees consistency and adherence among all teams, promoting a culture of accountability and trustworthiness in information management.
Electronic Data Capture (EDC): Leverage advanced EDC systems equipped with built-in validation checks. These technologies significantly minimize human errors and enhance precision, resulting in more dependable findings. The adoption of EDC systems has been shown to reduce study timelines by up to 30% compared to conventional methods, underscoring their transformative impact on clinical trials. As Jerry C Parker noted, "The adoption of EDC technologies is transforming the landscape of medical studies."
Information Backup and Recovery: Implement robust backup solutions to safeguard against information loss. Establishing effective recovery processes is crucial for maintaining information integrity and ensuring continuity in research activities.
Access Controls: Enforce strict access restrictions to limit information manipulation to authorized personnel only. This practice mitigates the risk of information tampering and enhances the overall security of sensitive material.
Change Management: Develop a formal change management process to document modifications to data or procedures. This guarantees transparency and traceability, which are vital for upholding adherence and integrity throughout the research lifecycle.
Common Pitfalls: Be aware of potential barriers to EDC adoption, such as regulatory compliance concerns, which can complicate implementation. Understanding these challenges can help organizations navigate the intricacies of information management more effectively.
By following these best practices, organizations can cultivate a culture of information reliability that permeates all operational levels, ultimately enhancing the credibility and trustworthiness of their clinical research efforts. For instance, the Indiana Department of Transportation (INDOT) improved asset oversight capabilities through standardized information collection methods, exemplifying the efficiency of organized information management practices.

Investing in thorough employee training is crucial for maintaining information accuracy. Effective training programs encompass several key components:
By emphasizing employee development, organizations empower their teams to maintain information accuracy, ultimately aiding in the achievement of successful research results.

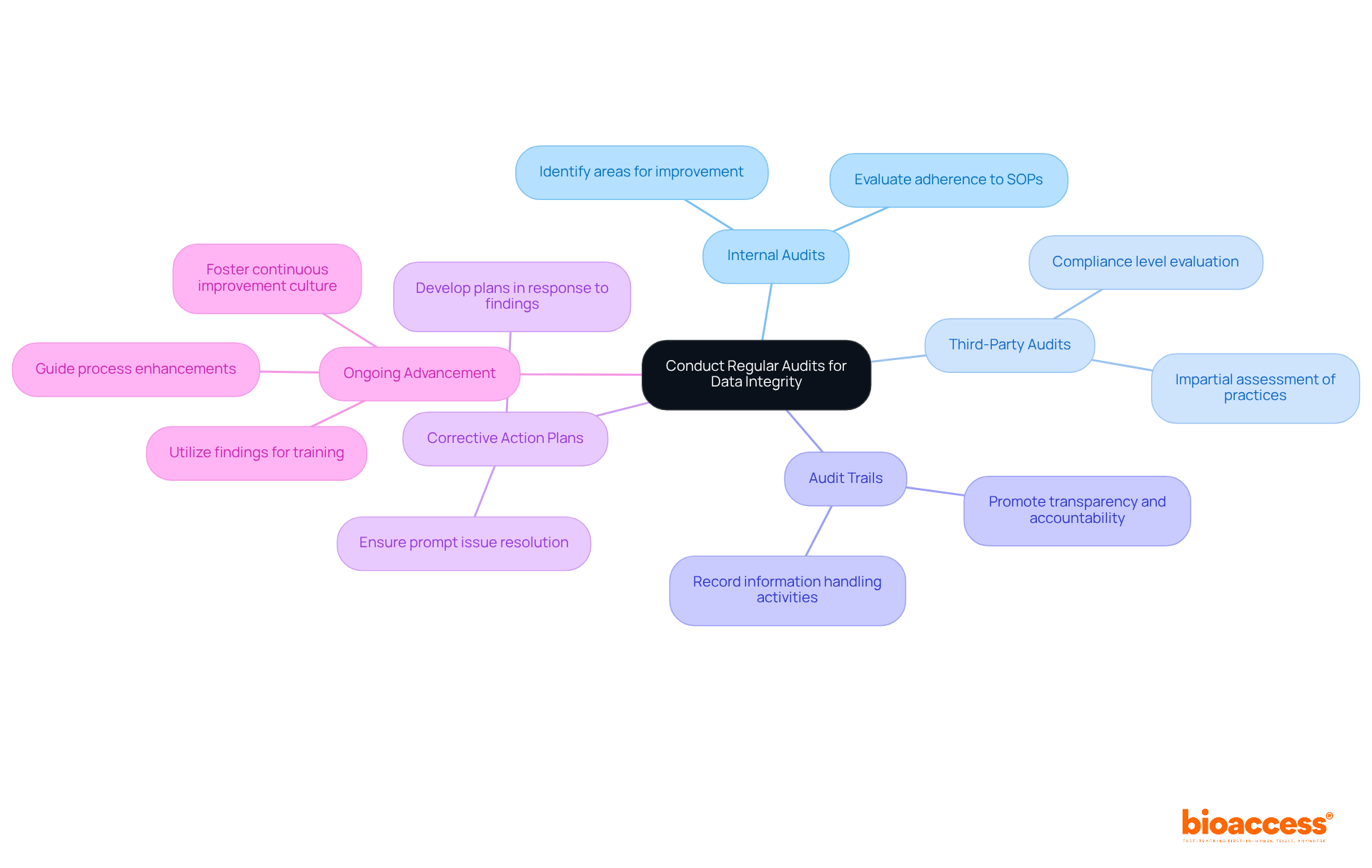
Carrying out regular audits is essential for ensuring data integrity in pharma company clinical research. Organizations must prioritize the following practices to enhance their operational integrity:
By integrating regular audits into their operational framework, organizations can ensure ongoing compliance and significantly enhance data integrity in pharma company clinical research.
Ensuring data integrity in the pharmaceutical industry transcends mere regulatory obligation; it is a fundamental component that underpins the reliability and credibility of research outcomes. By embracing the core principles of data integrity—Attributable, Legible, Contemporaneous, Original, and Accurate—organizations can establish a robust foundation for trustworthy information management. This unwavering commitment to data integrity is essential for navigating the complexities of compliance and fostering a culture of accountability within the industry.
The article delineates several best practices that organizations must implement to uphold data integrity and ensure compliance. These practices include:
Furthermore, investing in comprehensive staff training is vital, as it equips employees with the knowledge and skills necessary to maintain high standards of data accuracy and compliance. By actively engaging in these practices, pharmaceutical companies can significantly enhance the integrity of their clinical research efforts.
Ultimately, the significance of data integrity in the pharmaceutical sector cannot be overstated. It serves as a cornerstone of successful drug development and regulatory compliance, directly impacting patient safety and trust in healthcare systems. Organizations are urged to take proactive steps in implementing the discussed best practices, fostering a culture of continuous improvement, and committing to ongoing education and audits. By doing so, they will not only meet regulatory requirements but also contribute to the advancement of reliable and effective healthcare solutions.
What is the significance of data integrity in the pharmaceutical industry?
Data integrity in the pharmaceutical industry is crucial as it ensures the precision, consistency, and dependability of information throughout its lifecycle.
What does the acronym ALCOA stand for in the context of data integrity?
ALCOA stands for Attributable, Legible, Contemporaneous, Original, and Accurate. It serves as a fundamental guideline for ensuring the reliability of information.
Why is regulatory compliance important for data integrity in pharma?
Regulatory compliance is important because it mandates that information must be trustworthy and verifiable, as established by agencies such as the FDA and EMA.
What is meant by data lifecycle management?
Data lifecycle management refers to understanding the complete lifecycle of data—from collection to storage and analysis—to maintain quality at every stage.
How does transparency contribute to data integrity in pharma?
Transparency, through clear documentation and audit trails, fosters trust and accountability in the handling of information.
How can organizations uphold data integrity in their research outcomes?
Organizations can uphold data integrity by grasping and implementing the core principles of data integrity, which helps them navigate the complexities of information management effectively.