


Data integrity in the pharmaceutical industry is defined as the accuracy, consistency, and reliability of data throughout its lifecycle. This aspect is crucial for regulatory compliance and patient safety. The article emphasizes that adherence to key principles such as:
is essential. These principles help overcome challenges like human error and regulatory compliance, ultimately ensuring the production of safe and effective pharmaceutical products.
In an industry where precision can mean the difference between life and death, data integrity stands as a cornerstone of pharmaceutical research and development. Ensuring that information is accurate, consistent, and reliable is not merely a regulatory requirement; it is a fundamental necessity that safeguards patient safety and enhances the credibility of research outcomes. However, the journey to uphold data integrity is fraught with challenges, from human error to the complexities of regulatory compliance.
How can pharmaceutical companies navigate these hurdles while ensuring that their data remains trustworthy and verifiable?
The data integrity in the pharmaceutical industry is paramount, encompassing the accuracy, consistency, and dependability of information throughout its lifecycle, which includes collection, processing, storage, and reporting. In clinical trials, ensuring information accuracy is crucial; it guarantees that the produced details are reliable, enabling informed decisions regarding drug safety and effectiveness.
Data integrity in the pharmaceutical industry is essential for compliance with regulatory standards and for preserving the credibility of research findings, which directly influences patient safety and treatment outcomes. Experts emphasize that rigorous information management practices, including compliance reviews and thorough reporting, are vital to uphold data integrity in the pharmaceutical industry.
Any lapses can lead to significant challenges, such as information silos that obstruct collaboration and compromise patient safety. As the sector advances, incorporating cutting-edge technologies and adhering to established protocols, such as the FDA's ALCOA principles, will be critical in addressing these challenges and enhancing the overall quality of research information.
With bioaccess®'s extensive research project management services—including feasibility studies, site selection, compliance evaluations, project setup, import permits, project oversight, and reporting—the industry can significantly improve information accuracy and streamline processes.
Furthermore, the ability to recruit treatment-naive cardiology or neurology groups 50% quicker than Western locations, combined with achieving $25K savings per patient through FDA-ready information, underscores the substantial impact of effective clinical trial administration on both information quality and overall research outcomes.

The five principles of data integrity are essential for ensuring the reliability and accuracy of data in the pharmaceutical industry:
ALCOA: Data must be Attributable, Legible, Contemporaneous, Original, and Accurate. This principle ensures that information can be traced back to its origin and is documented clearly and swiftly, which is essential for regulatory compliance. As Hilary Mason states, "At the core of analytics and science is curiosity and learning; discovering patterns, narrating stories, and enhancing your comprehension of the world surrounding you."
Information Lifecycle Management: This principle emphasizes the importance of overseeing information throughout its lifecycle—from creation to destruction. Effective information lifecycle management is essential for maintaining data integrity in the pharmaceutical industry, ensuring that information remains precise, secure, and compliant at every stage, thereby minimizing risks associated with loss or corruption. Statistics indicate that organizations with strong information lifecycle management practices experience a 30% rise in compliance rates.
Quality Control: Implementing strong quality assurance measures is essential for maintaining data integrity in the pharmaceutical industry by identifying and rectifying mistakes in information collection and processing. This improves information reliability and upholds the trustworthiness of research results, which is essential in clinical studies. A case study from a prominent pharmaceutical firm demonstrated that enhanced quality control procedures decreased errors by 25%.
Training and Competence: Ensuring that all personnel involved in information handling are adequately trained and possess the necessary competencies is vital for maintaining integrity. Continuous education and training programs help staff stay updated on best practices and regulatory requirements. As pointed out by Jennifer Shin, "The information alone cannot provide all the answers you’re seeking."
Audit Trails: Maintaining comprehensive audit trails is essential for monitoring alterations made to information. This practice offers clarity and responsibility in information management, enabling organizations to show adherence to regulatory standards and internal policies.
By adhering to these principles, pharmaceutical firms can enhance their information reliability practices, thereby promoting data integrity in the pharmaceutical industry and ensuring the creation of safe, efficient, and high-quality pharmaceutical products.

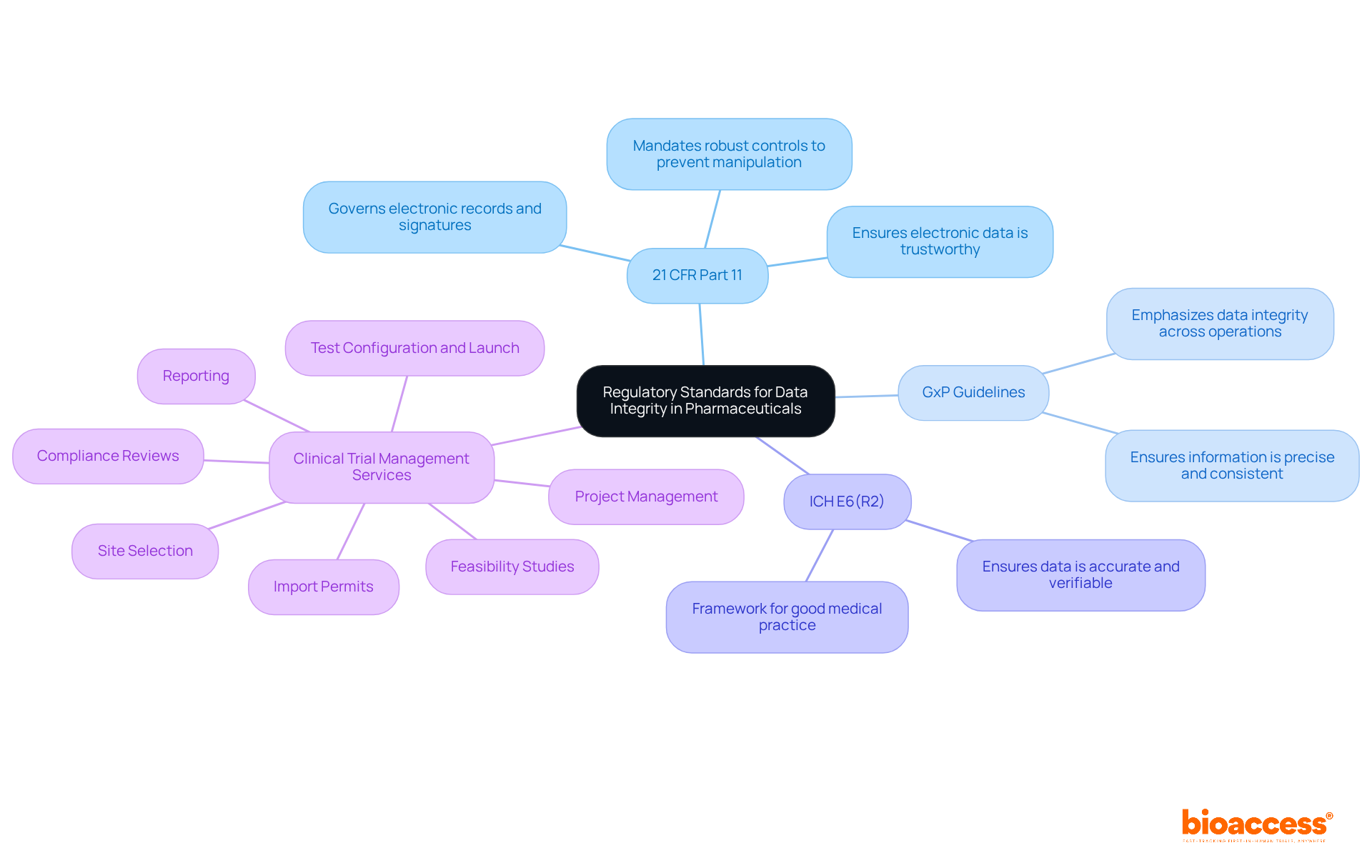
Regulatory standards for ensuring data integrity in the pharmaceutical industry are paramount, established by authorities such as the FDA, EMA, and ICH. These standards delineate expectations for information management practices in clinical trials, which are essential for maintaining data integrity in the pharmaceutical industry and safeguarding the reliability of records throughout the research process. Key regulations include:
21 CFR Part 11: This regulation governs electronic records and signatures, mandating that electronic data must be as trustworthy as traditional paper records. It underscores the necessity for robust controls to prevent manipulation of information and ensure data integrity in the pharmaceutical industry.
GxP Guidelines: Good Practice guidelines (GxP) encompass various facets of pharmaceutical operations, including research studies. They emphasize the importance of maintaining data integrity in the pharmaceutical industry throughout all procedures, ensuring that the information is precise, comprehensive, and consistent.
ICH E6(R2): This guideline outlines the framework for good medical practice, emphasizing the necessity of preserving data integrity in the pharmaceutical industry and ensuring that all data is accurate and verifiable. Adhering to these standards is crucial for achieving data integrity in the pharmaceutical industry, as they bolster the reliability of research outcomes and regulatory submissions for new medications and treatments.
At bioaccess, we understand that compliance with these standards is vital for the successful approval of new drugs and therapies. Our comprehensive clinical trial management services encompass:
By ensuring meticulous supervision and compliance with these standards, we protect data integrity in the pharmaceutical industry throughout the research process.
Maintaining data integrity in the pharmaceutical industry involves several challenges, such as human error, information security, the complexity of information management, regulatory compliance, and resource constraints.
Human Error: Mistakes in data entry or processing pose significant threats to data integrity. Research indicates that human error manifests through incorrect information entry, missed details, or failure to adhere to study protocols, leading to inaccuracies that undermine clinical trial results. As noted, 'Human error is one of the most frequent threats to information integrity.' Regular training and quality checks are essential to mitigate this risk, as ongoing staff education significantly reduces the likelihood of errors.
Information Security: Safeguarding information from unauthorized access or breaches is crucial. In 2023, healthcare information breaches reached an alarming rate of 1.99 incidents per day, with hacking accounting for nearly 80% of these breaches. Furthermore, it is vital to acknowledge that '32% of all documented breaches from 2015 to 2022 occurred in the healthcare sector,' underscoring the critical nature of breaches in this industry. Establishing robust cybersecurity protocols, such as encryption and two-factor authentication, alongside a security management procedure that includes a risk assessment and implemented remediation strategy, is essential to protect sensitive information and uphold information accuracy.
Complexity of Information Management: The increasing volume and complexity of information generated in research trials can lead to challenges in maintaining information integrity. Error rates in clinical research databases can vary significantly, with studies revealing discrepancies ranging from 2.3% to 26.9%. For example, the pooled error rate for single-data entry is 0.29%, while for double-data entry, it stands at 0.14%. Streamlined information management systems and processes, including the use of Electronic Information Capture (EDC) systems, can effectively address these challenges by enhancing data integrity in the pharmaceutical industry and facilitating real-time monitoring.
Regulatory Compliance: Keeping pace with evolving regulatory requirements can be daunting. Regulatory agencies such as the FDA and EMA mandate that information must be accurate, complete, and verifiable. Organizations must remain informed and adjust their practices accordingly to uphold compliance; neglecting to do so can result in severe penalties and jeopardize the reliability of research. Failures in information reliability may lead to the loss of clinical trial records, potentially incurring costs in the millions.
Resource Constraints: Limited resources can hinder the ability to implement thorough information reliability measures. A lack of training may result in unintentional errors or violations in information accuracy. Prioritizing data integrity within organizational strategy is vital to allocate appropriate resources effectively, ensuring that all personnel understand their responsibilities in maintaining data quality.

Data integrity stands as the cornerstone of the pharmaceutical industry, ensuring the accuracy, consistency, and reliability of information throughout its lifecycle. This principle is vital not only for regulatory compliance but also for patient safety and the integrity of research findings. By prioritizing data integrity, pharmaceutical companies foster trust and accountability in their processes, ultimately leading to safer and more effective therapeutic solutions.
The article explores the five essential principles of data integrity:
Each principle plays a crucial role in safeguarding data accuracy and reliability, underscoring the need for rigorous practices and continuous education in the face of evolving challenges. Moreover, compliance with regulatory standards such as 21 CFR Part 11 and GxP guidelines is imperative for maintaining data integrity and ensuring successful drug development.
Reflecting on the outlined challenges—including human error, information security threats, and resource constraints—it becomes evident that a proactive approach is necessary. Organizations must invest in robust training, advanced information management systems, and stringent compliance measures to navigate these complexities effectively. By doing so, the pharmaceutical industry can enhance data integrity and significantly improve research outcomes and patient care. Emphasizing the importance of data integrity transcends mere regulatory requirement; it embodies a commitment to excellence and responsibility within the healthcare sector.
What is data integrity in the pharmaceutical industry?
Data integrity in the pharmaceutical industry refers to the accuracy, consistency, and dependability of information throughout its lifecycle, including collection, processing, storage, and reporting.
Why is data integrity important in clinical trials?
Ensuring information accuracy in clinical trials is crucial as it guarantees that the produced details are reliable, enabling informed decisions regarding drug safety and effectiveness.
How does data integrity affect patient safety and treatment outcomes?
Data integrity is essential for compliance with regulatory standards and preserving the credibility of research findings, which directly influences patient safety and treatment outcomes.
What practices are necessary to uphold data integrity in the pharmaceutical industry?
Rigorous information management practices, including compliance reviews and thorough reporting, are vital to maintain data integrity in the pharmaceutical sector.
What challenges can arise from lapses in data integrity?
Lapses can lead to significant challenges such as information silos that obstruct collaboration and compromise patient safety.
What role do technologies and protocols play in enhancing data integrity?
Incorporating cutting-edge technologies and adhering to established protocols, such as the FDA's ALCOA principles, are critical in addressing challenges and enhancing the overall quality of research information.
How can bioaccess® help improve data integrity in pharmaceutical research?
Bioaccess® offers extensive research project management services, including feasibility studies, site selection, compliance evaluations, project setup, import permits, project oversight, and reporting, which significantly improve information accuracy and streamline processes.
What advantages does effective clinical trial administration provide?
Effective clinical trial administration can recruit treatment-naive cardiology or neurology groups 50% quicker than Western locations and achieve $25K savings per patient through FDA-ready information, impacting both information quality and overall research outcomes.