


This article delves into the essential process of database locking in clinical trials, highlighting its critical role in safeguarding data integrity and ensuring compliance. It provides a comprehensive step-by-step guide for executing a successful database lock, which includes:
These elements collectively bolster the reliability of trial outcomes and regulatory submissions.
In the ever-evolving Medtech landscape, understanding the intricacies of database locking is paramount. As clinical trials face increasing scrutiny, the need for robust data management practices becomes more pressing. By addressing these challenges, organizations can enhance their operational efficiency and maintain compliance with regulatory standards.
Collaboration stands at the forefront of effective database locking. Engaging various stakeholders not only streamlines the process but also fosters a culture of accountability and transparency. As we navigate the complexities of clinical research, it is crucial to recognize the importance of teamwork in achieving successful outcomes.
Locking a database in clinical trials is not merely a procedural step; it represents a critical milestone that safeguards the integrity and credibility of research findings. By ensuring the accuracy of collected data, a well-executed database lock not only supports regulatory compliance but also builds trust among stakeholders.
Yet, the complexities involved in achieving a successful lock prompt essential questions:
This guide explores the essential strategies and insights necessary for mastering the database lock process in clinical trials.
A database lock clinical trial serves as a pivotal milestone in clinical studies, marking the moment when the data repository is finalized and no further modifications can occur. This process is vital for safeguarding the accuracy and integrity of the data collected during the trial. The significance of a repository hold lies in its ability to protect the data from alterations, which is essential for compliance submissions and the overall credibility of trial outcomes. A well-executed data lock not only prepares the information for analysis but also builds trust among stakeholders regarding the research findings.
Recent studies highlight that a structured data closure process can significantly enhance data integrity, with effective collaboration between Clinical Data Management and Biostatistics teams being crucial. For instance, establishing clear criteria for securing data early in the process can accelerate the data lock finalization, reducing the risk of delays or errors. Furthermore, the average time from data closure to the completion of Clinical Study Reports (CSRs) is approximately 83 days, underscoring the importance of timely and accurate data management.
In summary, the database lock clinical trial hold is not merely a procedural formality; it serves as a cornerstone of clinical trial integrity, ensuring that the data is trustworthy and prepared for regulatory review.
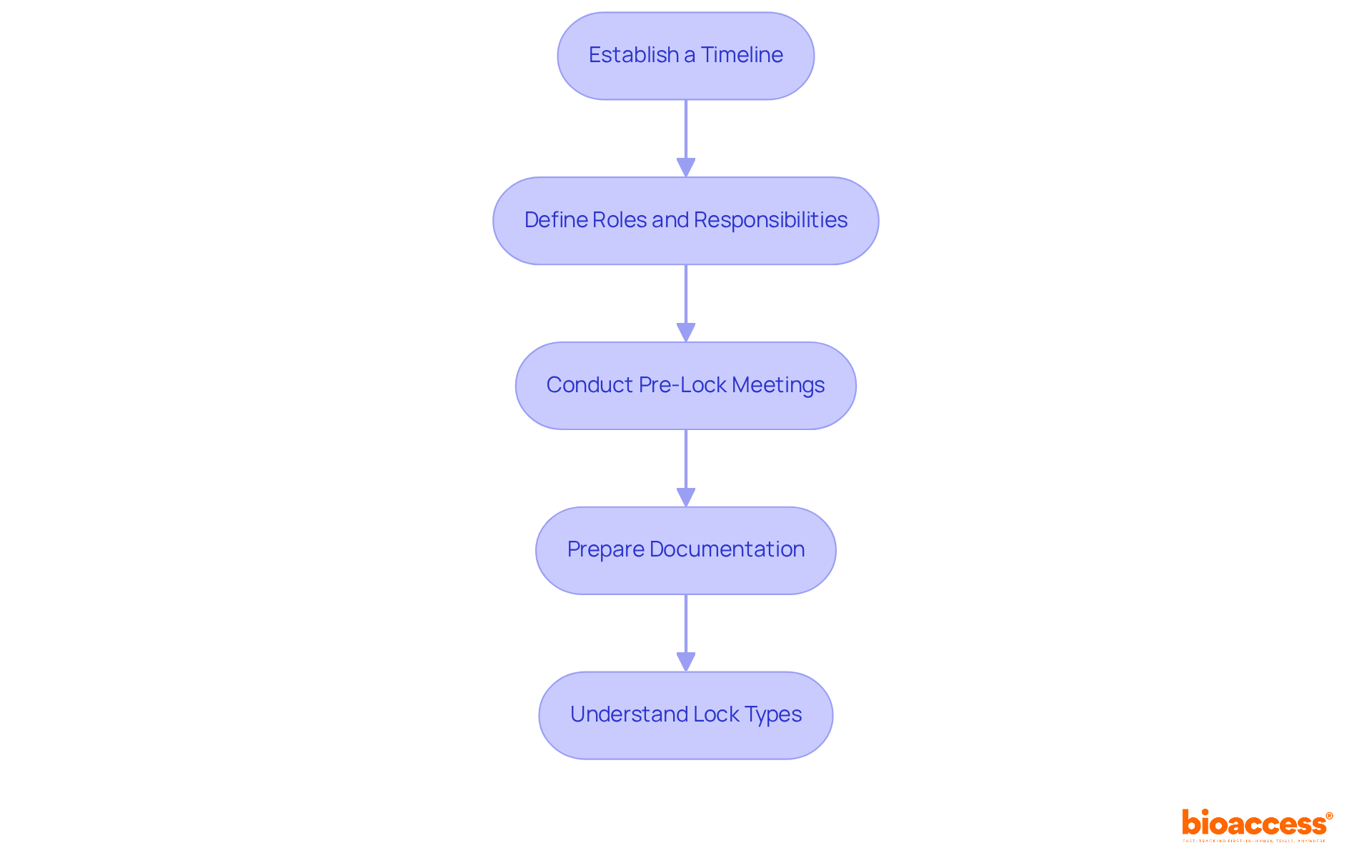
To ensure a successful database lock, developing a comprehensive plan is essential. This plan should encompass the following steps:
Establish a Timeline: Create a detailed timeline outlining all key milestones leading up to data closure. This includes specific deadlines for data cleaning, query resolution, and final reviews. Efficient project management with organized schedules is crucial, as delays in data closure can cost pharmaceutical firms between $600,000 and $8 million daily. This statistic highlights the importance of timely execution in the locking process.
Define Roles and Responsibilities: Clearly assign tasks to team members, ensuring everyone understands their duties leading up to the deadline. This clarity fosters focus and accountability, which are vital for a smooth locking process.
Conduct Pre-Lock Meetings: Schedule regular meetings to discuss progress, address issues, and ensure all team members are aligned with the plan. These meetings promote collaboration and proactive problem-solving, essential for timely database locking. Additionally, conducting pre-release activities such as final information cleaning and addressing queries is crucial for ensuring preparedness for closure.
Prepare Documentation: Ensure all required documentation, including information management plans and checklist reviews, is organized and accessible to the team. Proper documentation supports compliance and facilitates a seamless transition to the locking phase, reinforcing the integrity of the collected data. Including contingency planning in your strategy can help mitigate risks that might delay database closure, ensuring the team is ready for unexpected challenges.
Understand Lock Types: Familiarize the team with soft and hard barriers. A soft restriction is a temporary measure that limits edits but can be reversed if necessary, while a hard constraint is a final, irreversible barrier on the data, signaling readiness for final analysis or regulatory submission. Understanding these concepts is essential for efficiently managing the locking process.
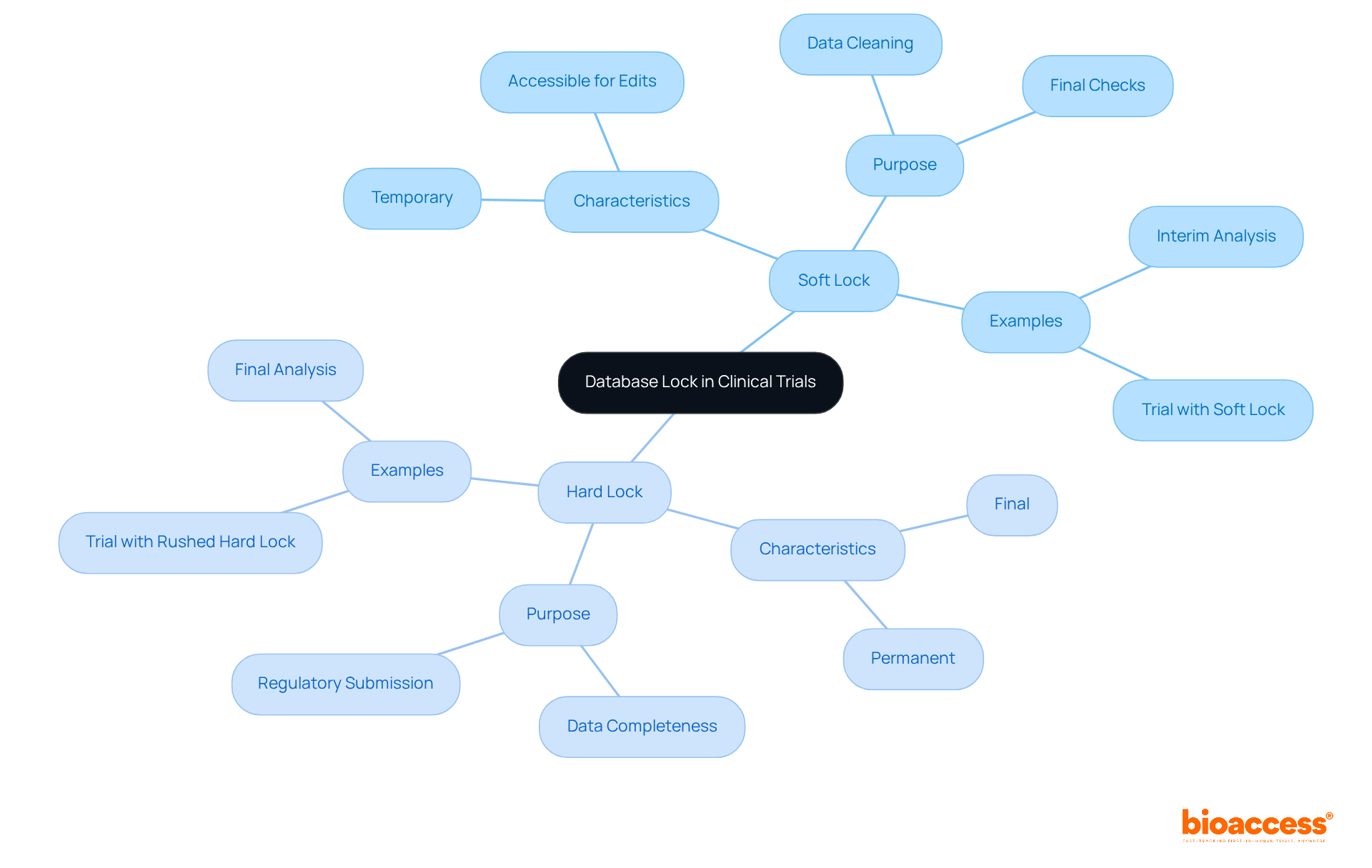
In clinical trials, understanding the database lock clinical trial is crucial for ensuring data integrity and precision. There are two primary types of locks:
Soft Lock: This temporary condition allows for final checks and information cleaning. During a soft lock, data remains accessible for querying and editing, enabling last-minute adjustments before the final lock is applied. This process is vital for resolving any outstanding issues, ensuring the integrity of the information.
Hard Lock: This represents the final locking stage, where the database is permanently closed to any further changes. A stringent safeguard indicates that the information is complete, verified, and ready for examination. Achieving this firm barrier is essential for preserving the validity of the study's results, as it prevents any modifications after the barrier is set.
Statistics indicate that a significant proportion of clinical trials utilize soft locks to facilitate comprehensive data review and guarantee accuracy before the database lock clinical trial is implemented. Expert insights underscore the importance of collaboration among Clinical Operations, Clinical Information Management, and Biostatistics teams during the locking process, which can greatly enhance data quality and decision-making.
Real-world examples illustrate the practical implications of these mechanisms. For instance, one trial effectively employed a soft lock, allowing for critical data modifications that ultimately improved the quality of the final analysis. Conversely, another study faced challenges due to a rushed hard lock, resulting in inconsistencies that could have been avoided with a more thorough soft locking procedure.
Understanding these locking mechanisms is essential for ensuring that data is effectively managed in a database lock clinical trial, ultimately supporting reliable results and compliance with regulations.
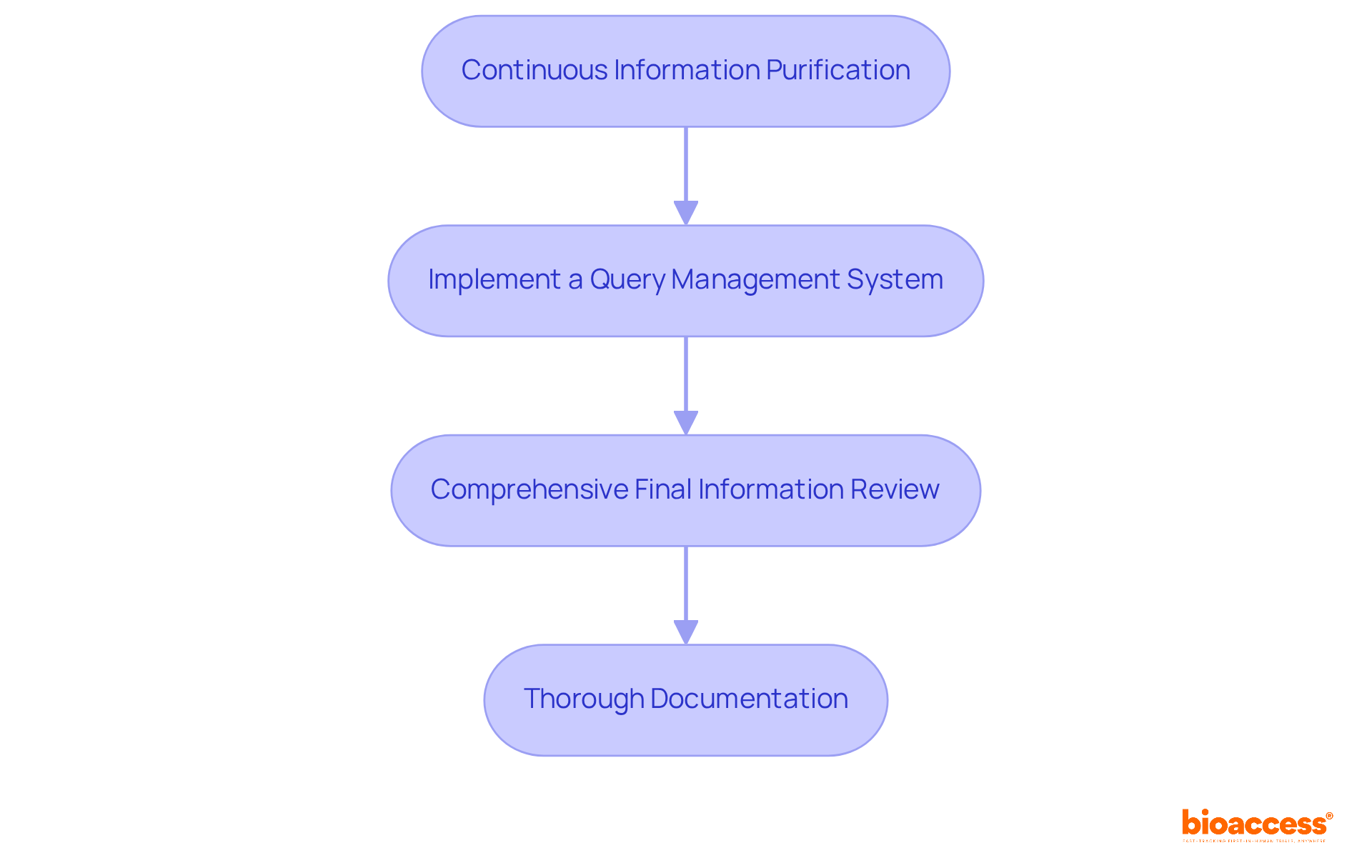
To ensure a seamless database lock, it’s crucial to follow these best practices for data cleaning and query resolution:
Continuous Information Purification: Engage in ongoing information cleansing throughout the study. This proactive approach allows for early detection of inconsistencies and verification of entries, significantly reducing the workload at the study's conclusion. The Society for Clinical Information Management highlights that regular information cleaning can cut down the time needed for query resolution, ultimately enhancing trial efficiency.
Implement a Query Management System: Create a structured system for identifying, tracking, and resolving queries. Keeping the team updated on the status of outstanding queries fosters accountability and transparency. A case study from Tinuiti shows that a centralized query management system improved their query resolution times by 30%, underscoring the effectiveness of systematic approaches.
Comprehensive Final Information Review: Before locking the database, conduct a thorough examination of the information. This includes cross-referencing entries with source documents and confirming that all queries have been addressed, ensuring the integrity of the data. Recent advancements in automated information review tools have proven effective in streamlining this process, enabling quicker identification of discrepancies.
Thorough Documentation: Maintain meticulous records of all data cleaning efforts and query resolutions. This documentation serves as a clear audit trail, essential for regulatory compliance and future reference. Experts in the field emphasize that comprehensive documentation not only aids in adherence but also enhances the reliability of results.
By incorporating these practices, clinical research teams can significantly improve the effectiveness of the information system closure process, leading to more dependable trial outcomes.
To enhance cross-functional collaboration during the database lock process, consider implementing the following strategies:
Regular Communication: Open lines of communication among all team members—clinical operations, information management, and biostatistics—are essential. Frequent updates and discussions help identify potential issues early, ensuring alignment and awareness. As Christine Redmond, vice president of clinical operations and program management for Ratio Therapeutics, emphasizes, "Everyone must understand the objectives for effective study design and planning."
Collaborative Tools: Utilize collaborative platforms that facilitate real-time information sharing and progress tracking. These tools streamline communication and enhance transparency across teams, which is crucial given the complexity of oncology studies, as noted by Redmond.
Joint Problem-Solving Sessions: Conduct sessions where team members collaboratively tackle challenges and brainstorm solutions. This approach fosters innovation and strengthens team dynamics, reinforcing commitment to shared goals. Involving biostatisticians early in the process is vital, as their participation ensures that all necessary information for analysis is collected and assessed.
Celebrate Milestones: Acknowledge and celebrate key milestones in the locking process. Recognizing achievements fosters a sense of teamwork and accomplishment, motivating team members to continue working effectively together.
By prioritizing these communication strategies, clinical trial teams can significantly enhance the effectiveness of the information securing process, ultimately leading to more successful trial outcomes. Thorough planning from the study's inception is also vital for a successful database lock, as highlighted in best practices for data cleaning and query resolution.
A well-executed database lock stands as a cornerstone of clinical trials, marking the definitive moment when data is finalized and protected from alterations. This critical process not only safeguards the integrity and accuracy of the data but also instills confidence among stakeholders regarding the trial's findings. Recognizing the significance of this milestone is vital for maintaining compliance and delivering credible results.
Key insights from this guide underscore the necessity of a structured approach to database locking:
These are pivotal steps in ensuring a seamless locking process. Furthermore, implementing best practices for data cleaning and fostering cross-functional collaboration can greatly enhance the efficiency and reliability of the data management process.
Ultimately, the success of a database lock relies on meticulous planning and teamwork. By prioritizing effective communication and collaboration among clinical operations, data management, and biostatistics teams, the integrity of clinical trial outcomes can be preserved. As the landscape of clinical trials continues to evolve, mastering the database lock process will remain fundamental to achieving trustworthy and impactful results.
What is a database lock in clinical trials?
A database lock in clinical trials is a critical milestone that indicates the finalization of the data repository, preventing any further modifications to the data collected during the trial.
Why is a database lock important in clinical trials?
A database lock is important because it safeguards the accuracy and integrity of the trial data, ensuring compliance for submissions and enhancing the credibility of the trial outcomes.
How does a structured data closure process benefit clinical trials?
A structured data closure process enhances data integrity by fostering effective collaboration between Clinical Data Management and Biostatistics teams, which can expedite the data lock finalization and minimize the risk of delays or errors.
What is the average time from data closure to the completion of Clinical Study Reports (CSRs)?
The average time from data closure to the completion of Clinical Study Reports (CSRs) is approximately 83 days.
What are the key steps to plan for a successful database lock?
Key steps include establishing a timeline, defining roles and responsibilities, conducting pre-lock meetings, preparing documentation, and understanding lock types.
Why is establishing a timeline crucial for a database lock?
Establishing a timeline is crucial because it outlines key milestones leading to data closure, helping to avoid costly delays, which can amount to between $600,000 and $8 million daily for pharmaceutical firms.
What roles should be defined in the database locking process?
Roles should be clearly assigned to team members to ensure everyone understands their tasks leading up to the deadline, promoting focus and accountability.
What is the purpose of conducting pre-lock meetings?
Pre-lock meetings serve to discuss progress, address issues, and ensure all team members are aligned with the plan, fostering collaboration and proactive problem-solving.
What documentation is necessary for a successful database lock?
Necessary documentation includes information management plans and checklist reviews, which support compliance and facilitate a smooth transition to the locking phase.
What are the differences between soft and hard database locks?
A soft lock is a temporary restriction that limits edits but can be reversed, while a hard lock is a final, irreversible barrier on the data, indicating readiness for final analysis or regulatory submission.