


This article presents best practices for design and validation in clinical research, underscoring the critical role of systematic processes in ensuring that medical devices not only meet user needs but also comply with stringent regulatory standards. It offers a thorough overview of effective methodologies, including:
These approaches collectively enhance product reliability and safety, ultimately contributing to improved patient outcomes. The insights provided herein are essential for navigating the complex Medtech landscape, where addressing key challenges requires a collaborative effort among all stakeholders.
In the intricate landscape of clinical research, the design and validation process stands as a cornerstone for ensuring that medical solutions not only meet user needs but also comply with stringent regulatory standards. This process is vital for unlocking the potential for safer, more effective products that resonate with end users.
By delving into best practices for design validation, researchers can significantly enhance their approaches. However, the challenge remains: how can teams effectively integrate stakeholder feedback and navigate complex regulatory environments to enhance their validation strategies? This question is crucial in driving forward the efficacy of clinical research.
The design and validation process in clinical research serves as a systematic method to ensure a solution meets the requirements of its intended users while adhering to regulatory standards. This involves a series of activities aimed at confirming that outputs align with established inputs, which include user needs and intended use. The FDA mandates that design assessments be conducted under actual or simulated use conditions, guaranteeing that the product performs as expected in real-world scenarios. Such a rigorous process is essential for minimizing risks and protecting patient safety, which is crucial for achieving successful clinical outcomes.
For example, a medical device intended for patient monitoring must undergo comprehensive testing to validate its ability to accurately capture and transmit data across various conditions, mirroring the real environments in which it will be deployed. This thorough verification not only strengthens product reliability but also aligns with FDA guidelines, which emphasize the importance of design and validation documentation to ensure adherence and facilitate regulatory approval.
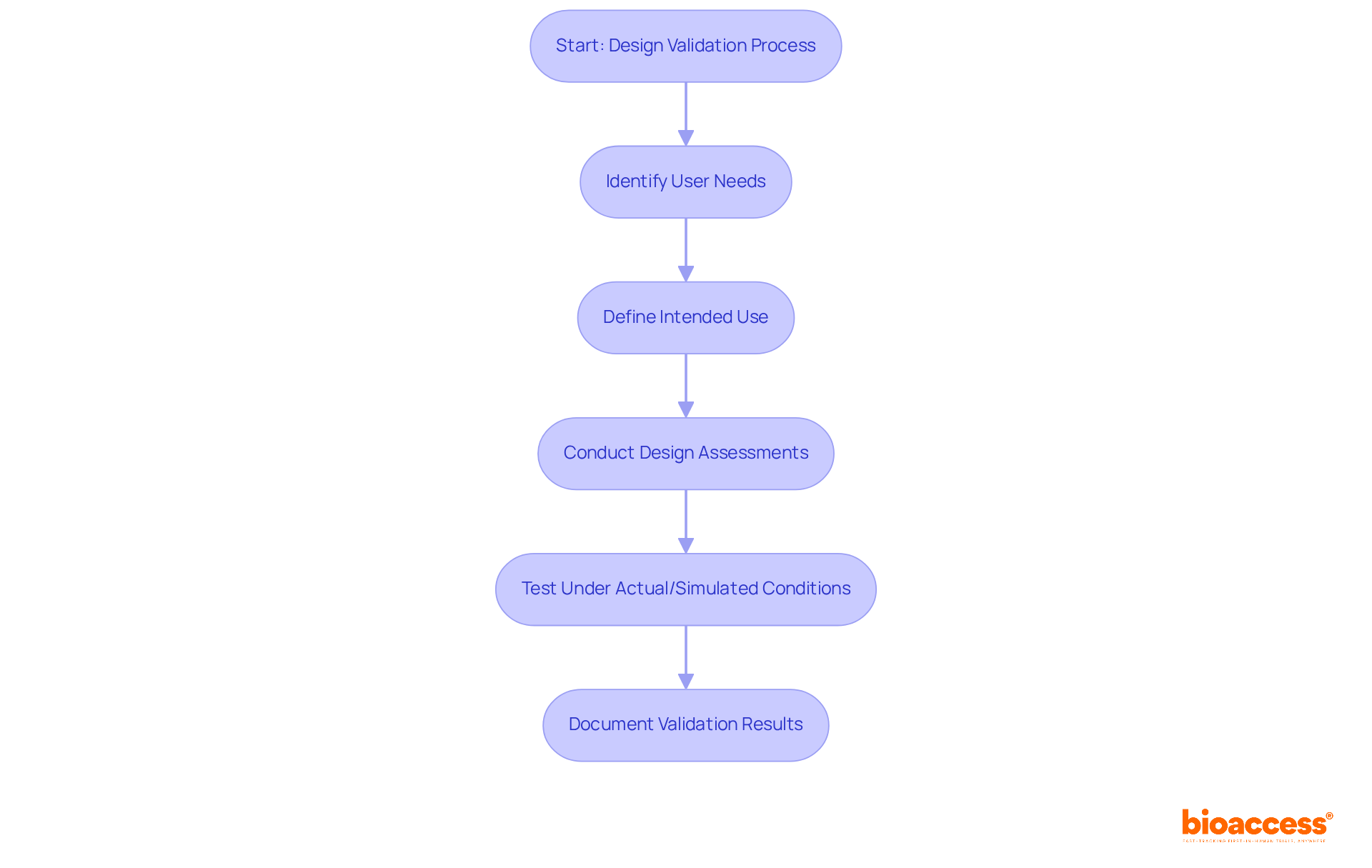
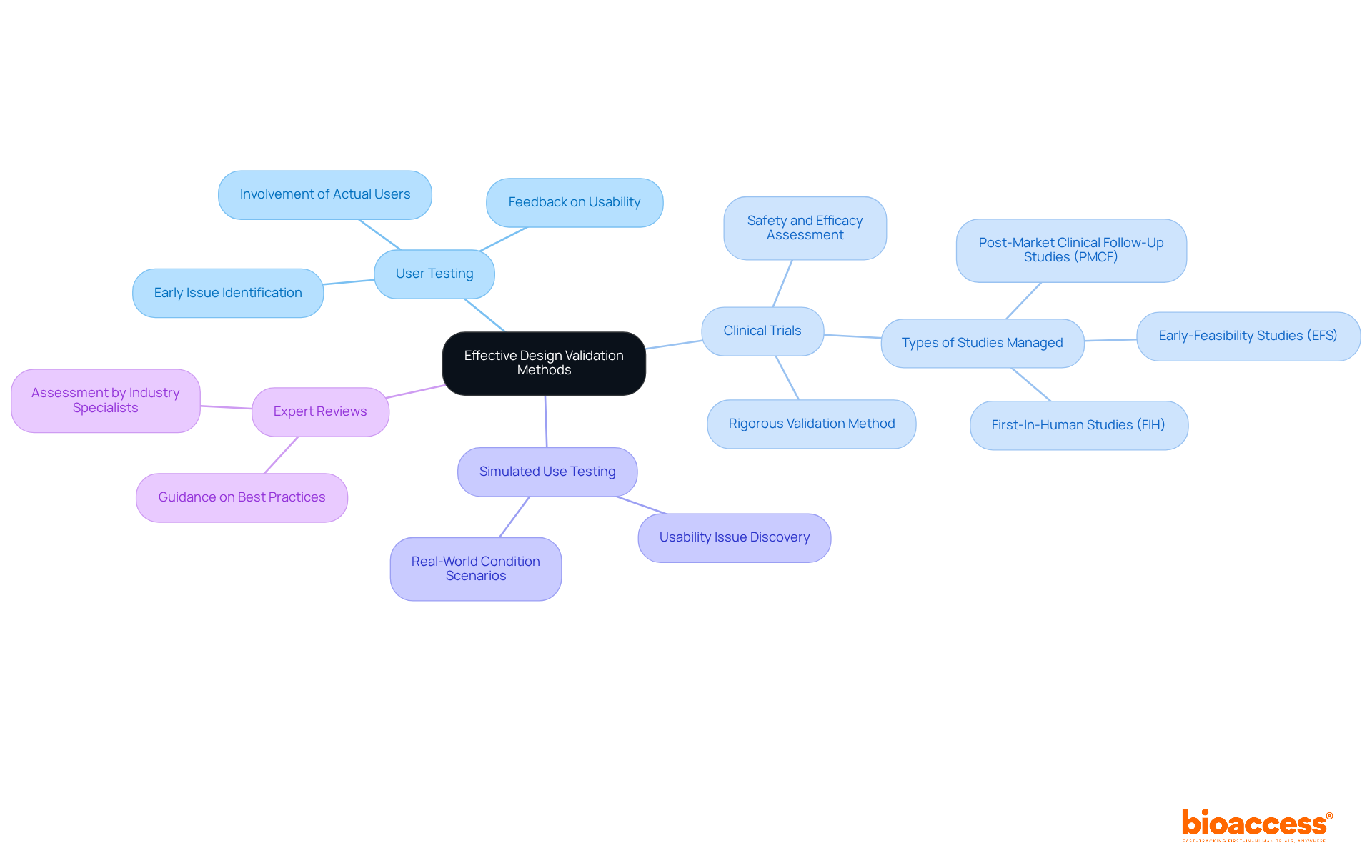
Effective design and validation in clinical research can be achieved through several key methods that ensure both usability and compliance with regulatory standards.
User Testing involves actual users in the testing process, providing valuable feedback on usability and functionality. This approach helps identify potential issues early, ensuring that the design aligns with user expectations and needs.
Clinical Trials serve as a rigorous validation method that assesses the safety and efficacy of the item in a controlled environment. This method produces strong data on the item's performance, which is essential for regulatory approval. At bioaccess®, we specialize in managing various types of clinical studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and Post-Market Clinical Follow-Up Studies (PMCF). Our services encompass feasibility and selection of research sites, compliance reviews, trial setup, and effective project management, ensuring adherence to local regulations throughout the trial process.
Simulated Use Testing creates scenarios that replicate real-world conditions, allowing researchers to assess how the item performs under various circumstances. This method is particularly effective for uncovering usability issues that may not surface in traditional laboratory settings.
Expert Reviews involve industry specialists assessing the layout, offering perspectives derived from their vast experience and understanding of best practices. Their feedback can guide improvements and ensure compliance with regulatory standards.
Employing a mix of these techniques guarantees a comprehensive design and validation process that meets both user needs and regulatory standards, ultimately enhancing the item's success in the market.
Involving stakeholders—including patients, healthcare providers, and regulatory bodies—is essential for the successful design and validation of concepts during medical device development. Effective engagement is crucial for ensuring that products meet real-world challenges and stakeholder needs. Here are best practices for achieving this engagement:
Conduct Surveys and Interviews: Gathering insights from potential users about their needs, preferences, and experiences with similar offerings is vital. This data directs development choices and ensures the product effectively addresses real-world challenges.
Establish Advisory Boards: Forming advisory groups composed of diverse stakeholders provides continuous input during the development phase. This collaborative approach fosters a sense of ownership and ensures that various viewpoints are integrated into the creation process.
Iterative Prototyping: Creating prototypes and gathering user input at various stages of development allows for ongoing enhancements based on user feedback. Research shows that integrating user feedback can significantly improve product development success rates, with patient-engaged research reaching enrollment goals 25% quicker and undergoing 40% fewer protocol changes.
Clear Communication: Maintaining open lines of communication with stakeholders is essential to keep them informed about changes and how their feedback is being utilized. This transparency fosters trust and promotes continual cooperation, which is crucial for effective clinical research planning.
By actively involving stakeholders and integrating their feedback, researchers can improve the design and validation of their innovations, ultimately leading to enhanced patient outcomes. As industry experts emphasize, meaningful patient involvement transforms research from something done to patients into something done with them, ensuring that the resulting products truly meet their needs.

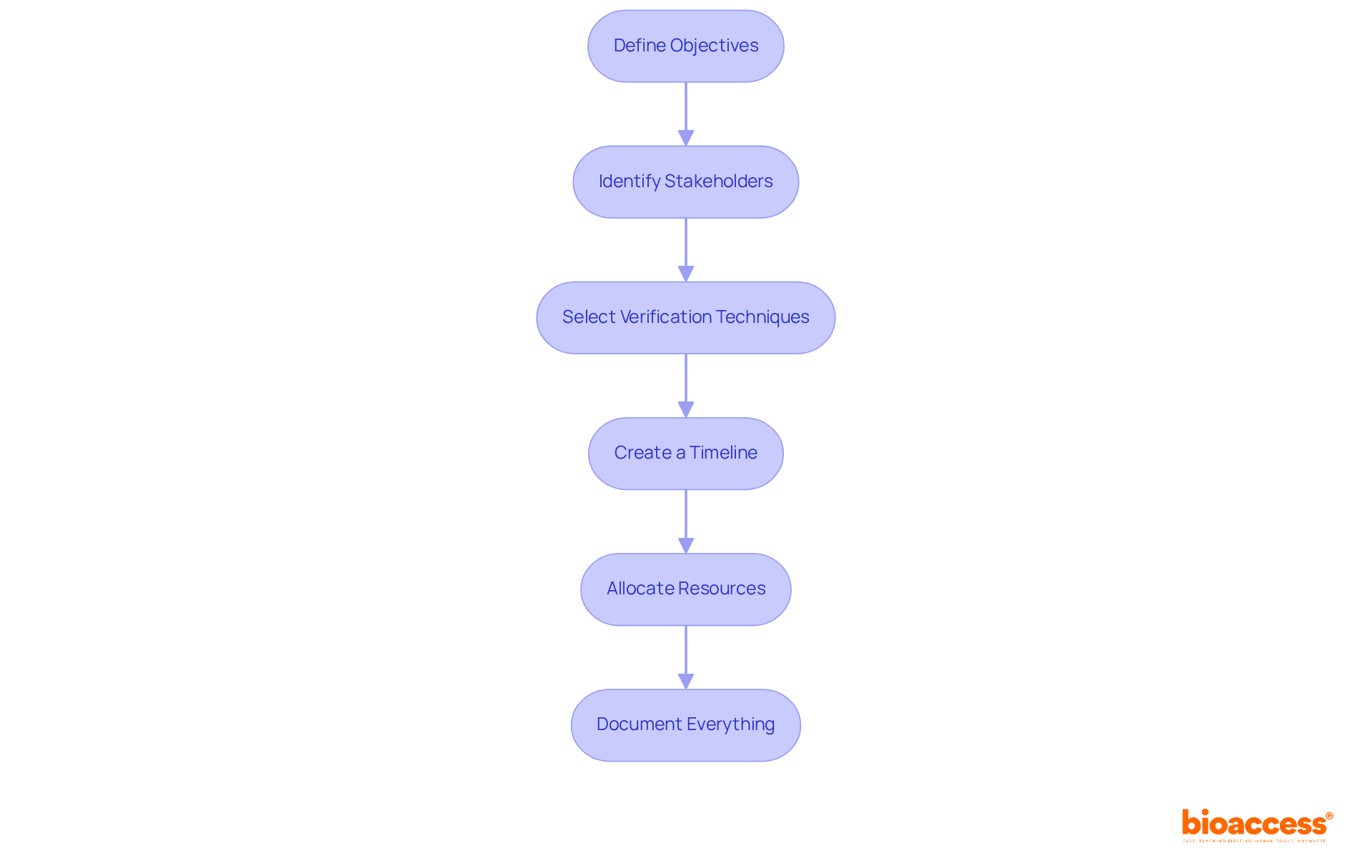
Developing a comprehensive design validation plan is essential in clinical research and involves several key components:
Define Objectives: Clearly outline the goals of the assessment, including what needs to be confirmed and the criteria for success.
Identify Stakeholders: List all parties involved in the assessment, including users, regulatory bodies, and team members. Define their roles and responsibilities.
Select Verification Techniques: Choose suitable verification techniques based on the product type and user needs. This may include user testing, clinical trials, and expert reviews. In the context of Latin America, leveraging bioaccess®'s expertise in clinical trial management, including feasibility studies and project management, can enhance the effectiveness of these methods, ensuring compliance with local regulations and addressing specific market needs.
Create a Timeline: Formulate a schedule for the verification procedure, incorporating milestones and due dates for each stage. This helps ensure that the project stays on track and meets regulatory timelines, particularly in navigating the complexities of the Latin American Medtech landscape.
Allocate Resources: Identify the resources needed for the verification process, including personnel, budget, and materials. Ensure that sufficient resources are allocated to support the verification activities, particularly when taking into account the distinct challenges and opportunities in the region.
Document Everything: Maintain thorough documentation of all verification activities, including test results, feedback, and changes made based on user input. This documentation is crucial for regulatory compliance and future reference, particularly in light of the oversight provided by authorities like INVIMA in Colombia.
By following these steps, researchers can create a comprehensive design and validation plan that effectively addresses all necessary components, ensuring a successful validation process while capitalizing on the support and resources available through organizations like bioaccess®.
The design and validation process in clinical research is crucial for ensuring that medical products not only meet regulatory standards but also address user needs effectively. By systematically confirming that outputs align with user expectations and intended use, researchers can minimize risks and enhance patient safety. This rigorous approach is not merely a regulatory requirement from bodies like the FDA; it is a vital component for achieving successful clinical outcomes.
Key insights from the article emphasize the significance of employing effective design validation methods, including:
Engaging stakeholders, such as patients and healthcare providers, enriches the development process by incorporating diverse perspectives and feedback. This collaborative effort ultimately results in products that are better equipped to tackle real-world challenges, thereby improving patient outcomes and ensuring regulatory compliance.
In light of these practices, it is imperative for researchers and organizations involved in clinical research to prioritize the formulation of comprehensive design validation plans. By clearly defining objectives, selecting suitable verification techniques, and maintaining meticulous documentation, the validation process can be streamlined and rendered more effective. Embracing these best practices not only boosts the likelihood of regulatory approval but also cultivates innovation that genuinely meets the needs of patients and healthcare professionals alike.
What is the purpose of design validation in clinical research?
The purpose of design validation in clinical research is to ensure that a solution meets the requirements of its intended users while adhering to regulatory standards.
What activities are involved in the design validation process?
The design validation process involves a series of activities aimed at confirming that outputs align with established inputs, including user needs and intended use.
What does the FDA require regarding design assessments?
The FDA requires that design assessments be conducted under actual or simulated use conditions to ensure that the product performs as expected in real-world scenarios.
Why is the design validation process important for patient safety?
The design validation process is important for minimizing risks and protecting patient safety, which is crucial for achieving successful clinical outcomes.
Can you provide an example of design validation in practice?
An example is a medical device intended for patient monitoring, which must undergo comprehensive testing to validate its ability to accurately capture and transmit data across various conditions that mimic real environments.
How does design validation relate to FDA guidelines?
Design validation strengthens product reliability and aligns with FDA guidelines that emphasize the importance of design and validation documentation to ensure adherence and facilitate regulatory approval.