


The primary focus of this article centers on the critical importance of mastering design control practices in alignment with FDA regulations, which is essential for the success of medical devices. It underscores that implementing a systematic design control framework not only elevates product quality and safety but also streamlines compliance with regulatory standards. This, in turn, leads to improved patient outcomes and mitigates risks associated with medical devices.
The landscape of medical device development is increasingly shaped by stringent regulatory frameworks and the necessity for safety and efficacy. Mastering design control practices set forth by the FDA is not merely a regulatory obligation; it is a critical factor in ensuring that medical devices meet user needs while minimizing risks.
Organizations face the complex challenge of navigating compliance, prompting the question: how can they effectively integrate robust design control processes to enhance product quality and expedite market entry?
This article delves into essential practices of design control, comparing FDA and ISO requirements, and outlines key steps for successful implementation, ultimately guiding manufacturers toward medical device success.
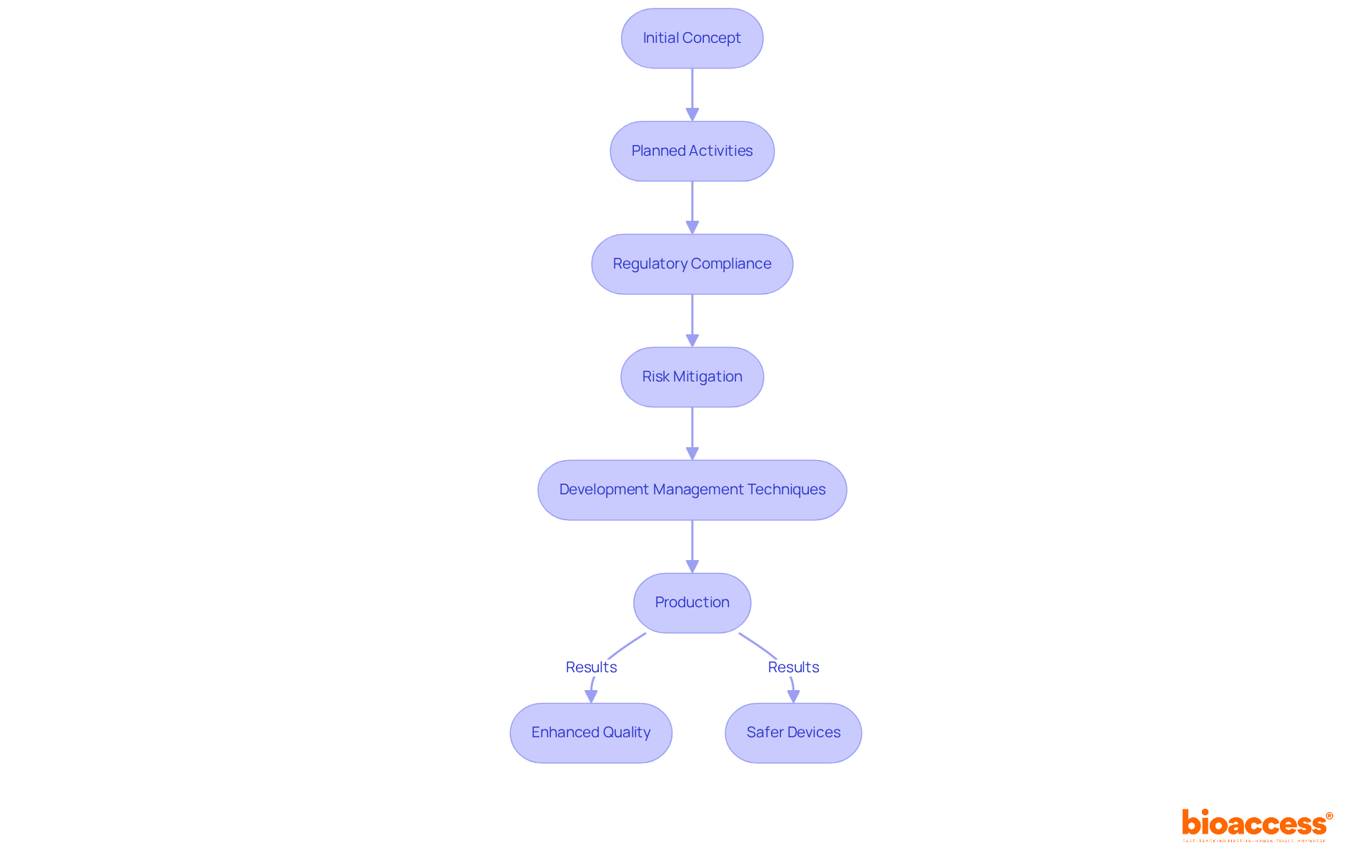
The management of creation serves as a systematic framework for design control FDA that guides the formulation and development of medical devices, ensuring they align with user needs and regulatory standards. This comprehensive process encompasses a series of planned and documented activities, extending from the initial concept to production. The significance of regulation in design control FDA is underscored by its capacity to mitigate risks associated with medical devices, thereby ensuring their safety and effectiveness for patient use. For instance, a meticulously organized plan for managing processes has been demonstrated to enhance project efficiency and product quality, as evidenced by case studies that reveal improved adherence to regulatory standards and better patient outcomes.
Recent advancements in design control FDA have further solidified their role within the medical device sector. By implementing robust development management techniques, organizations can not only refine their creation processes but also significantly reduce time to market. This proactive strategy results in enhanced product quality and compliance, ultimately yielding safer medical devices that satisfy the stringent requirements of regulatory bodies. Moreover, the integration of innovative guidelines into the development process is essential for achieving market success and ensuring that medical devices effectively fulfill their intended functions.
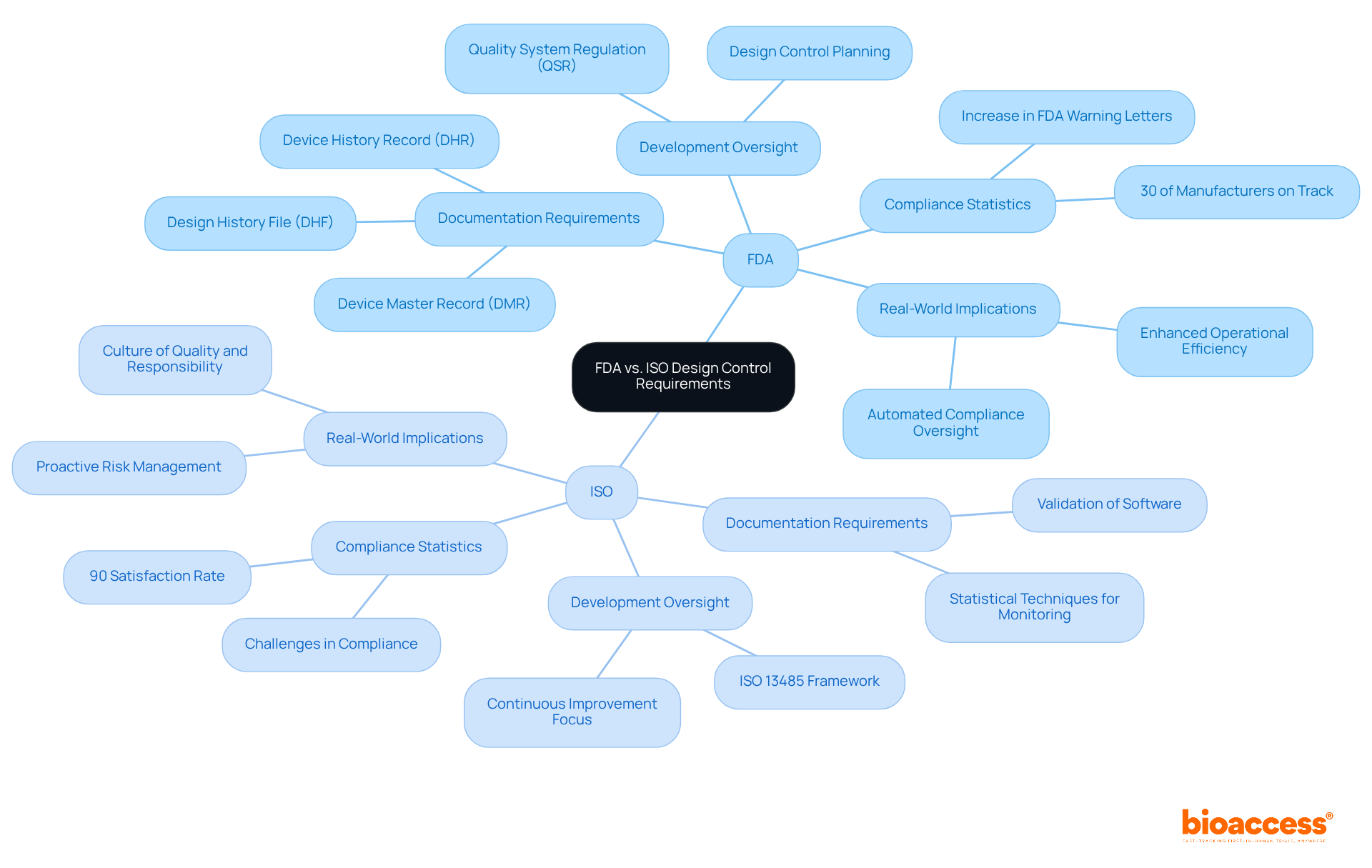
The FDA and ISO standards both emphasize the essential function of development oversight, yet they differ significantly in their specific requirements. The FDA's Quality System Regulation (QSR) mandates that manufacturers implement design control FDA to establish a comprehensive framework for managing the planning, input, output, review, verification, validation, and transfer of the layout. This structured approach is crucial for ensuring that medical devices meet safety and efficacy standards. In contrast, ISO 13485 incorporates development oversight within a broader quality management system framework, placing a strong emphasis on continuous improvement and risk management throughout the product lifecycle.
Recent statistics reveal that only 30% of manufacturers are currently on track to meet the upcoming QMSR adherence deadline. This statistic highlights the considerable challenges many organizations face in achieving compliance. Manufacturers are afforded a two-year transition period to align with the QMSR, commencing in February 2024, which underscores the urgency for organizations to grasp the distinct documentation and procedural requirements of both frameworks. For instance, while the FDA necessitates thorough documentation of modifications and validation activities, ISO 13485 promotes a culture of quality and responsibility. This necessitates organizations to meticulously record verification and validation strategies that incorporate statistical methods.
Real-world examples further illustrate the implications of these differences. Businesses that have successfully navigated FDA compliance concerning product development have reported enhanced operational efficiency and a reduction in errors, particularly through the implementation of automated compliance oversight systems. Effective documentation strategies are essential for sustaining adherence rates and facilitating smoother transitions to the new standards. Conversely, organizations that fail to align their procedures with design control FDA and ISO standards risk heightened scrutiny and potential regulatory action. This is evidenced by the increase in FDA warning letters, which surged from 17 in the early 2000s to 304 in 2020.
In summary, while both the FDA and ISO frameworks aim to ensure the safety and efficacy of medical devices, organizations must remain vigilant in adhering to the specific requirements of each framework. This diligence is critical for facilitating successful product development and ensuring timely market entry.
To effectively implement design control in medical devices, organizations must adhere to several key steps:
A pertinent case study is the 'Automation of Cytogenetics and FISH Workflows,' which demonstrates how organized development procedures resulted in enhanced turnaround times and superior quality outcomes in a laboratory environment.
Following these steps not only simplifies development oversight but also increases the chances of successful product creation and market entry, ultimately aiding in better patient outcomes and ensuring compliance with design control fda.

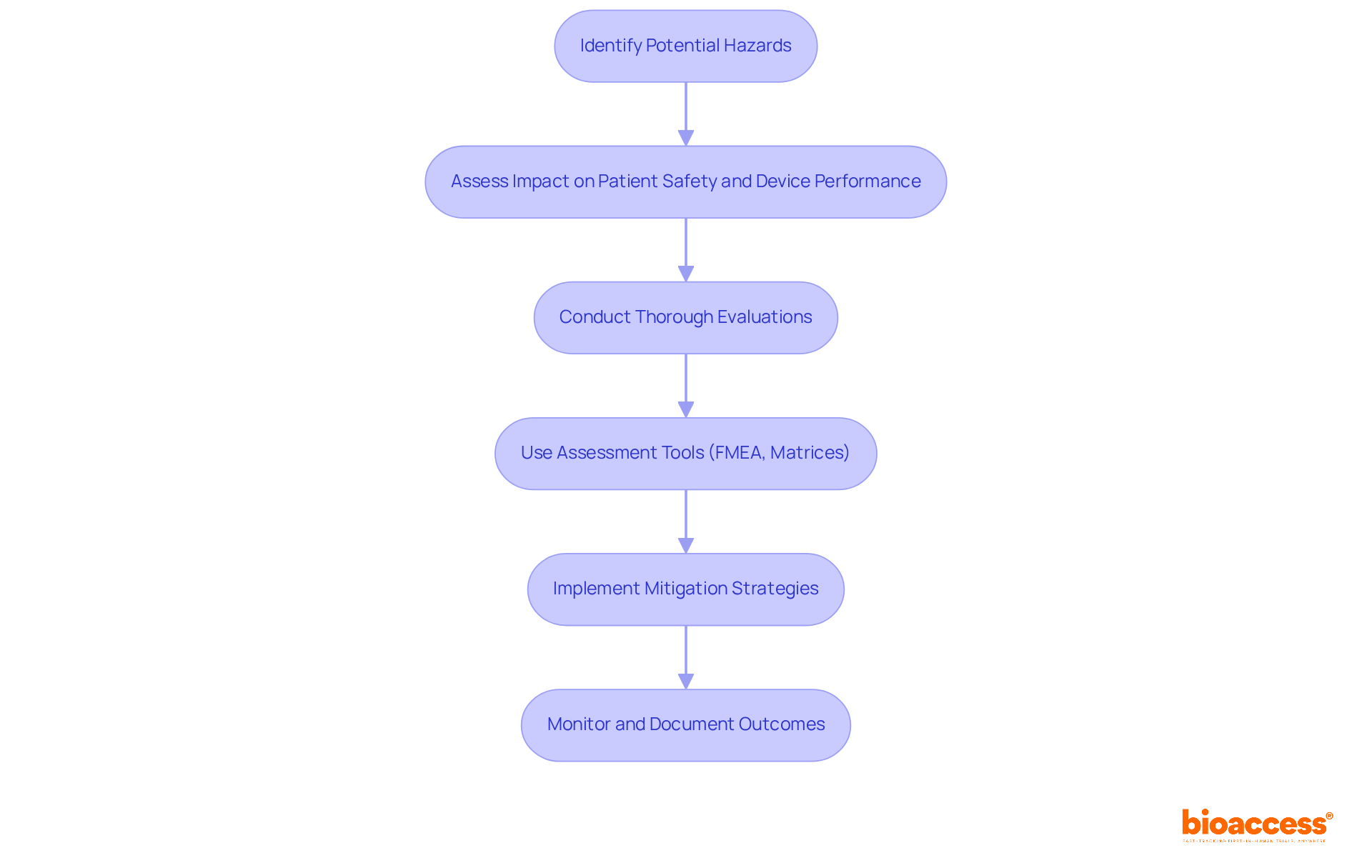
Incorporating hazard management into the control framework is essential for ensuring the safety and efficacy of medical devices. This process involves identifying potential hazards and assessing their impact on patient safety and device performance. Organizations must conduct thorough evaluations of potential issues at various stages of the development cycle, employing tools such as Failure Mode and Effects Analysis (FMEA) and quantitative assessment matrices to systematically analyze uncertainties. A study highlights that effective management practices can significantly reduce adverse events, illustrating a direct correlation between comprehensive assessments and improved safety statistics in medical device usage.
By meticulously documenting identified hazards and implementing appropriate mitigation strategies, organizations can integrate safety factors into the development phase. This proactive approach not only enhances compliance with regulatory requirements, such as those set forth by the FDA and ISO 13485, but also fosters a culture of safety and quality within the organization. Furthermore, the incorporation of advanced hazard assessment tools, including control software, improves the evaluation process, ensuring that all potential threats are managed effectively.
Practical examples underscore the importance of evaluations in medical device creation. For instance, manufacturers that adopted a systematic hazard management framework reported a notable decrease in product recalls and safety incidents. This emphasizes the necessity of prioritizing risk assessments as a critical aspect of the design control FDA process, ultimately resulting in safer and more reliable medical devices.
The effective management of design control practices is paramount for the success of medical devices, ensuring they meet both user needs and regulatory standards. By adopting a systematic approach to design control, organizations can significantly enhance product quality and safety, ultimately leading to improved patient outcomes. The integration of robust development management techniques streamlines the creation process and reduces the time required to bring medical devices to market, underscoring the critical importance of adhering to design control FDA practices.
Key insights from the article elucidate the distinctions between FDA and ISO design control requirements, highlighting the necessity for manufacturers to navigate these frameworks with care. By implementing essential steps in the design control process—such as:
Organizations can effectively mitigate potential hazards and ensure compliance with regulatory expectations. The significance of documentation and proper evaluation cannot be overstated, as these practices are fundamental to achieving successful product development and timely market entry.
In a landscape where compliance is non-negotiable, organizations are urged to prioritize the integration of design control and risk management into their development processes. By doing so, they not only enhance the safety and efficacy of their medical devices but also cultivate a culture of quality and accountability within their teams. Embracing these practices will safeguard patient health and position manufacturers for success in an increasingly competitive market.
What is design control in the context of medical devices?
Design control refers to a systematic framework that guides the formulation and development of medical devices, ensuring they meet user needs and regulatory standards.
Why is design control important for medical devices?
Design control is important because it helps mitigate risks associated with medical devices, ensuring their safety and effectiveness for patient use. It also enhances project efficiency and product quality.
What activities are involved in the design control process?
The design control process includes a series of planned and documented activities that extend from the initial concept to production.
How does design control impact project efficiency and product quality?
A meticulously organized design control plan has been shown to improve adherence to regulatory standards and lead to better patient outcomes, thereby enhancing project efficiency and product quality.
What recent advancements have been made in design control for medical devices?
Recent advancements include the implementation of robust development management techniques that refine creation processes and significantly reduce time to market, resulting in enhanced product quality and compliance.
How does design control contribute to market success for medical devices?
The integration of innovative guidelines into the development process is essential for achieving market success, ensuring that medical devices effectively fulfill their intended functions and meet regulatory requirements.