


Understanding the complexities of device feasibility study coordination in Romania is crucial for navigating the rapidly evolving clinical research landscape. With the market for clinical trials projected to expand significantly, stakeholders must grasp the regulatory frameworks, recruitment strategies, and collaborative approaches that underpin successful studies. The challenge, however, lies in effectively integrating these elements to ensure compliance, enhance participant engagement, and ultimately drive better patient outcomes.
How can stakeholders leverage effective practices to not only meet regulatory demands but also foster innovation and inclusivity in their research efforts? This question invites a deeper exploration into the Medtech landscape and highlights the role of bioaccess in addressing key challenges. Collaboration and strategic planning are essential as we move forward in this dynamic environment.
Understanding the regulatory frameworks governing clinical research is crucial for successful device feasibility study coordination in Romania. The National Agency for Medicines and Medical Devices (NAMMD) oversees the approval process, ensuring compliance with European Union regulations and Good Clinical Practice (GCP) standards. Familiarity with these regulations not only streamlines approvals but also protects patient welfare and data integrity. Key regulations include:
These regulations outline the necessary documentation, ethical considerations, and timelines for project initiation.
Notably, the NAMMD guarantees a maximum approval duration of 60 days for new research study applications, while the Romanian Regulatory Agency has a maximum of 30 days to confirm or reject an authorization request. Engaging with local regulatory specialists, such as those at bioaccess, can enhance compliance and simplify the approval process. This collaboration ultimately leads to quicker project initiation and improved patient outcomes. Bioaccess® offers comprehensive trial management services that encompass device feasibility study coordination in Romania, along with:
This ensures that Medtech, Biopharma, and Radiopharma startups can expedite their trials and secure regulatory approval effectively.
As Romania's clinical research market is projected to grow from €72 million in 2019 to over €210 million by 2026, grasping these frameworks becomes increasingly vital for stakeholders looking to navigate this evolving landscape successfully.

Implementing effective participant recruitment strategies is crucial for the success of device feasibility study coordination in Romania. With a diverse population of approximately 19.4 million, it’s vital to tailor recruitment efforts to resonate with various cultural and demographic groups. Strategies may include:
Understanding the specific health concerns and preferences of different populations can significantly enhance engagement. For instance, conducting focus groups to gather insights on perceptions can guide recruitment messaging and improve participation rates. Collaborating with local advocacy groups builds trust and promotes enrollment, ultimately resulting in a more representative research sample.
Notably, Romania has over 500 active clinical trials, with a major focus on oncology, neurology, and gastroenterology. This underscores the necessity for effective recruitment strategies customized for various demographics, particularly in the realm of device feasibility study coordination in Romania, ensuring that research efforts are both inclusive and impactful.

Encouraging collaboration with local stakeholders is crucial for successful device feasibility study coordination in Romania. Engaging with healthcare experts, regulatory agencies, and advocacy organizations provides invaluable insights and support throughout the device feasibility study coordination in Romania. By forming partnerships with local hospitals and clinics, recruitment efforts can be bolstered, and data collection can be significantly improved.
Bioaccess stands out by offering comprehensive trial management services, including trial setup, project oversight, and expedited participant recruitment. With over 50 pre-qualified sites activated in under eight weeks, the efficiency of trials is greatly enhanced. Furthermore, maintaining open lines of communication with regulatory bodies ensures compliance with all requirements, effectively reducing the risk of delays. Regular meetings and updates with stakeholders address concerns and foster a collaborative atmosphere, ultimately leading to more effective project execution and improved patient outcomes.
As Ali Cundari, Senior Director of Marketing and Corporate Communications, aptly states, "In 2025, sustainability in research is a shared responsibility and an opportunity for innovation." This perspective highlights the growing trend of decentralized clinical trials (DCTs), which significantly mitigate the environmental impact of clinical research and influence stakeholder participation in feasibility assessments. It is also essential to recognize common pitfalls in stakeholder engagement, such as inadequate communication or unclear roles, which can hinder project success.

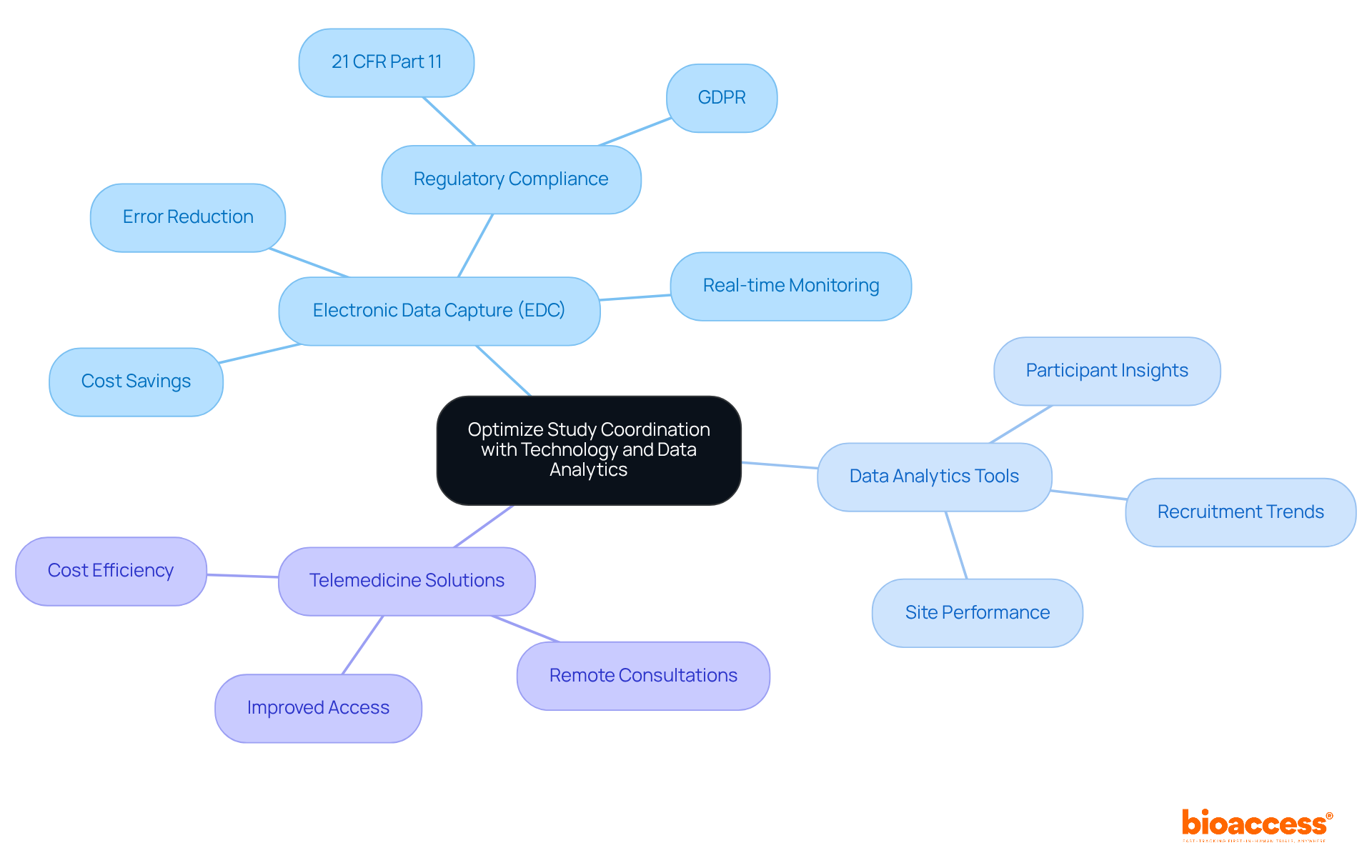
Harnessing technology and data analytics is crucial for enhancing device feasibility study coordination in Romania during feasibility assessments. Electronic data capture (EDC) systems streamline data collection, significantly reducing errors and improving real-time monitoring of study progress. The successful implementation of EDC systems in Romania highlights their capacity to boost operational efficiency and ensure compliance with regulatory standards, including 21 CFR Part 11 and GDPR, under the device feasibility study coordination in Romania. Moreover, data analytics tools offer valuable insights into participant demographics, recruitment trends, and site performance, enabling informed decision-making that can enhance trial outcomes.
The integration of telemedicine solutions facilitates remote patient consultations, improving access and convenience for participants. By adopting these technologies, clinical research teams can not only cut costs but also elevate the quality of study outcomes. This ultimately advances the field of clinical research in Romania, emphasizing the importance of device feasibility study coordination in Romania, along with collaboration and innovation in overcoming key challenges.
Understanding the complexities of device feasibility study coordination in Romania is crucial for stakeholders who want to effectively navigate the evolving landscape of clinical research. By grasping the regulatory frameworks, implementing targeted patient recruitment strategies, fostering collaboration with local stakeholders, and leveraging technology, researchers can significantly enhance the efficiency and success of their studies.
Key insights from this discussion highlight the necessity of adhering to regulations set forth by the National Agency for Medicines and Medical Devices (NAMMD), which streamline approval processes. Tailored recruitment strategies ensure diverse population representation, while collaboration with local experts enriches data collection and project execution. Moreover, integrating technology and data analytics optimizes study coordination, providing actionable insights that enhance trial outcomes.
Ultimately, the future of device feasibility studies in Romania relies on a multifaceted approach that embraces regulatory compliance, community engagement, and technological innovation. By adopting these best practices, stakeholders can not only expedite their research efforts but also contribute to the advancement of clinical research in Romania, paving the way for improved patient outcomes and groundbreaking medical advancements.
Why is understanding regulatory frameworks important for feasibility studies in Romania?
Understanding regulatory frameworks is crucial for successful device feasibility study coordination in Romania as it ensures compliance with European Union regulations and Good Clinical Practice (GCP) standards, streamlining approvals and protecting patient welfare and data integrity.
Which organizations oversee the approval process for clinical research in Romania?
The National Agency for Medicines and Medical Devices (NAMMD) oversees the approval process for clinical research in Romania.
What are the key regulations that govern clinical research in Romania?
The key regulations include the Medical Devices Regulation (MDR) and the Clinical Trials Regulation (CTR), which outline necessary documentation, ethical considerations, and timelines for project initiation.
What is the maximum approval duration for new research study applications in Romania?
The NAMMD guarantees a maximum approval duration of 60 days for new research study applications, while the Romanian Regulatory Agency has a maximum of 30 days to confirm or reject an authorization request.
How can engaging with local regulatory specialists benefit the approval process?
Engaging with local regulatory specialists can enhance compliance and simplify the approval process, leading to quicker project initiation and improved patient outcomes.
What services does Bioaccess® offer for device feasibility study coordination in Romania?
Bioaccess® offers comprehensive trial management services that include feasibility assessments, site selection, compliance evaluations, trial preparation, import permits, project oversight, and progress reporting.
What is the projected growth of Romania's clinical research market?
Romania's clinical research market is projected to grow from €72 million in 2019 to over €210 million by 2026.
Why is grasping regulatory frameworks becoming increasingly vital for stakeholders in Romania?
Grasping regulatory frameworks is becoming increasingly vital for stakeholders as the clinical research market evolves, allowing them to navigate the landscape successfully and expedite their trials and regulatory approvals.