


This article delivers a comprehensive step-by-step guide for clinical research directors on effectively preparing, submitting, and maintaining Drug Master Files (DMFs) to ensure compliance with FDA regulations. It underscores the critical importance of DMF filing for safeguarding proprietary information and streamlining regulatory processes. Essential steps are detailed, including:
Navigating the intricate world of Drug Master Files (DMFs) is essential for clinical research directors who seek to streamline regulatory submissions while safeguarding proprietary information. DMFs are a crucial asset in the pharmaceutical industry, ensuring that sensitive data remains confidential while fostering essential communication with the FDA. However, the complexity of DMF filing presents significant challenges, often resulting in costly delays if not executed with precision.
How can clinical research directors master the DMF filing process to enhance compliance and efficiency in their submissions?
A DMF filing serves as a confidential submission to the FDA, providing comprehensive information about the facilities, procedures, and materials involved in the production, handling, packaging, and storage of human drug products. DMF filing is essential for safeguarding proprietary data while facilitating regulatory submissions, allowing companies to present vital details without disclosing sensitive information to the public. This confidentiality is crucial for maintaining competitive advantages within the pharmaceutical landscape.
Key components of a DMF filing include:
By organizing DMFs effectively, companies can streamline their regulatory processes, ensuring compliance with FDA requirements. For example, the process of DMF filing is integral to Investigational New Drug Applications (INDs), New Drug Applications (NDAs), and Abbreviated New Drug Applications (ANDAs), underscoring their indispensable role in the drug approval process.
As we look ahead to 2025, the importance of DMF filing in clinical research continues to grow. They not only protect proprietary data but also enhance the efficiency of regulatory evaluations with proper DMF filing. Statistics reveal that improper formatting or incomplete submissions can result in significant delays in FDA reviews, highlighting the necessity for thorough preparation. Regulatory experts assert that DMF filing is primarily reviewed when referenced in applications, emphasizing its strategic value in the regulatory landscape. By mastering the purpose and structure of DMFs, clinical research directors can establish a solid foundation for compliance and successful submissions.

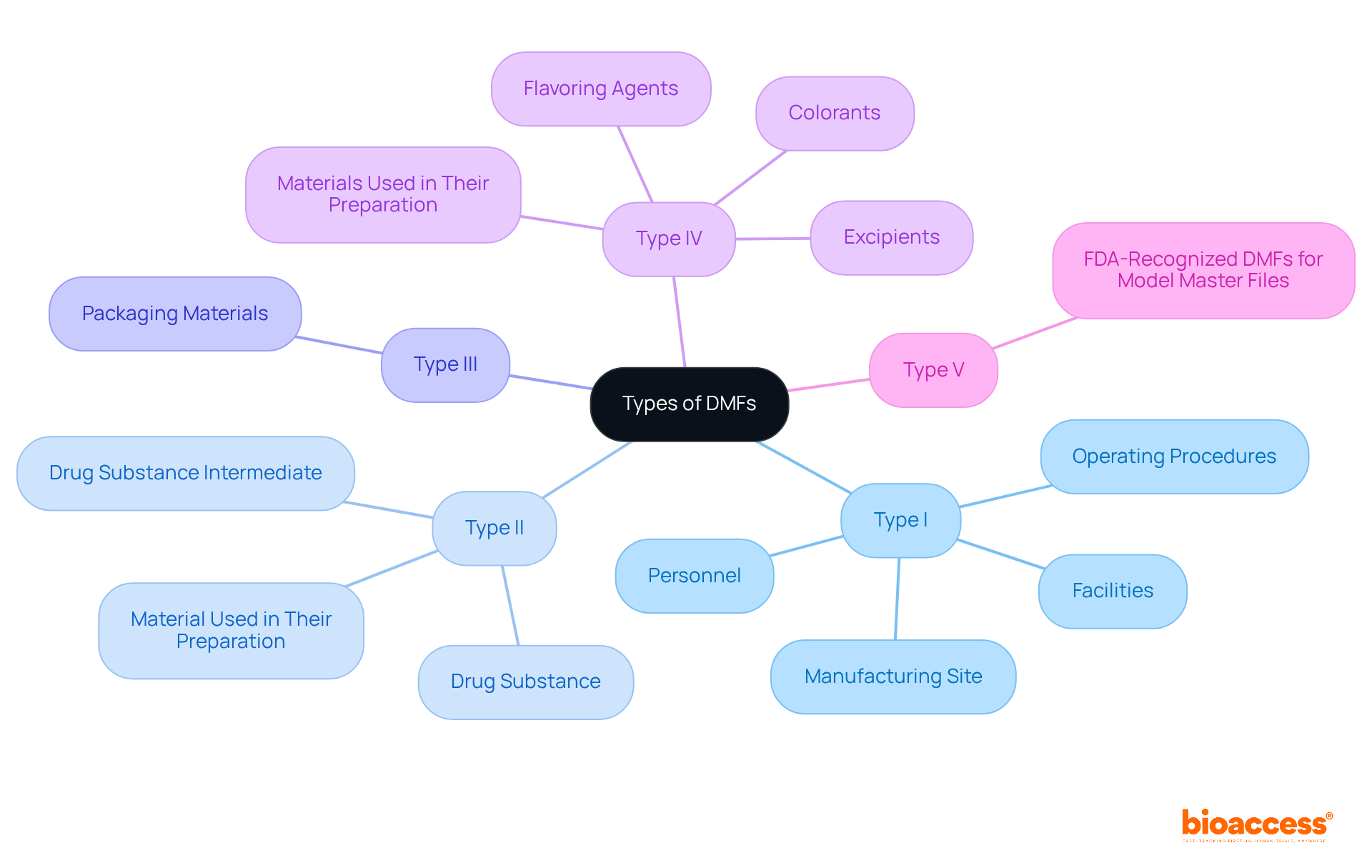
The FDA recognizes five primary types of DMFs, each serving a distinct purpose:
Each category fulfills a particular function and contains unique details. Understanding these types is crucial for aligning your DMF with regulatory expectations, ensuring that your submission is both comprehensive and compliant.
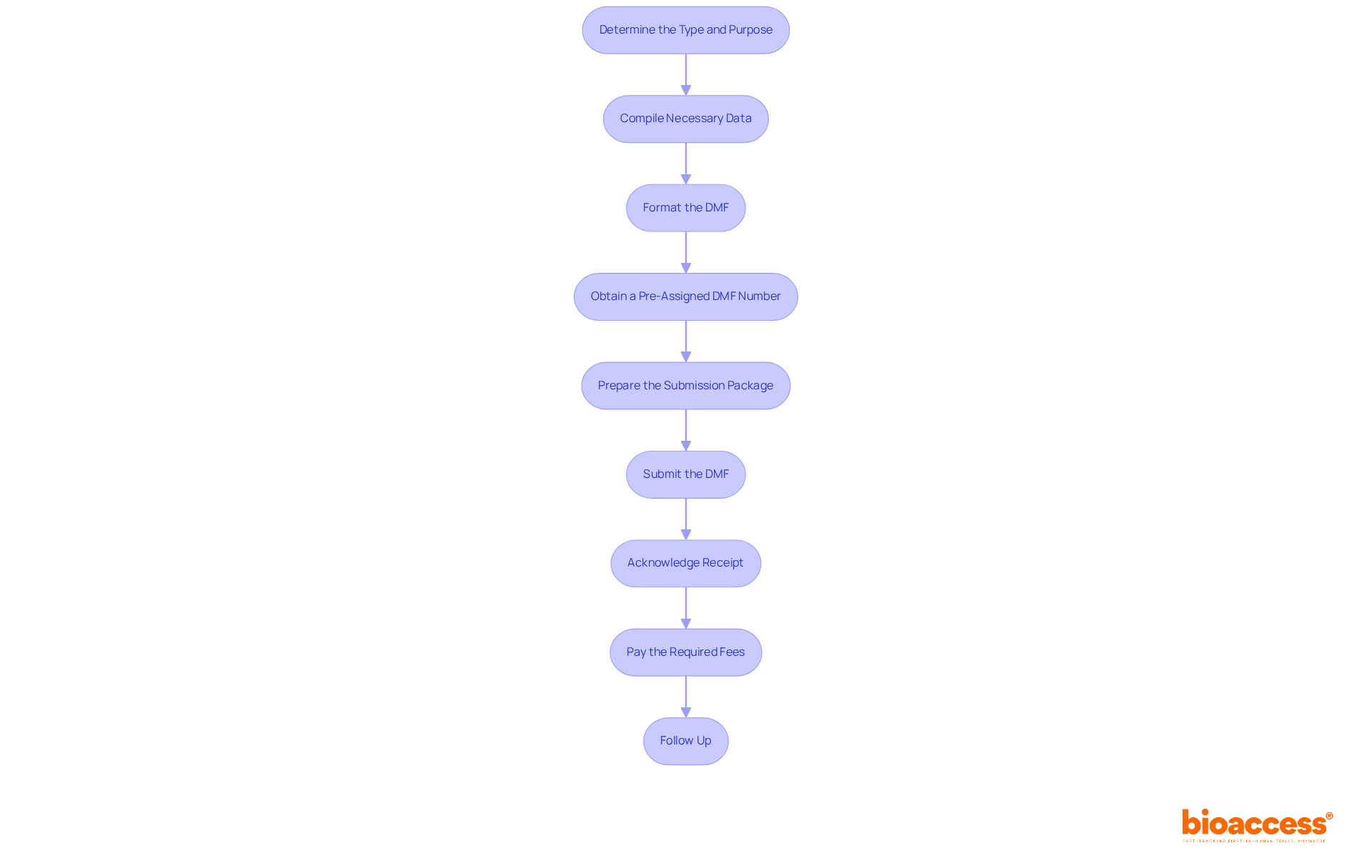
To prepare and submit your DMF filing, follow these essential steps:
Engaging with specialists such as Ana Criado, a leader in regulatory affairs with extensive experience, and Katherine Ruiz, a professional in regulatory affairs for medical devices and in vitro diagnostics, can provide invaluable insights during this process, ensuring compliance and efficiency.
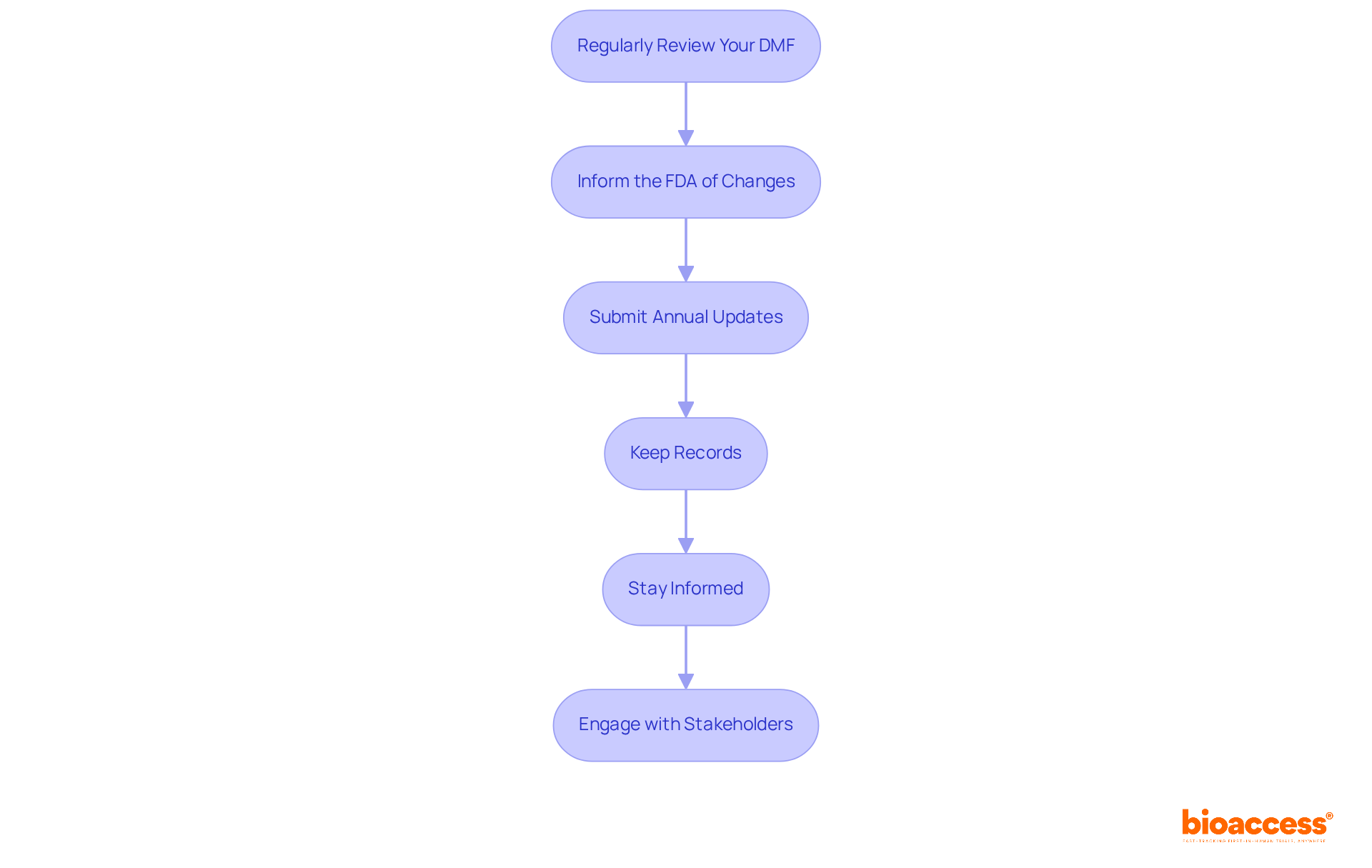
To effectively maintain and update your DMF, consider the following essential steps:
Mastering the Drug Master File (DMF) filing process is essential for clinical research directors navigating the complex landscape of pharmaceutical regulations. Understanding the intricacies of DMFs—including their purpose, types, and submission requirements—enables professionals to safeguard proprietary information while ensuring compliance with FDA standards. This strategic approach not only enhances the chances of successful drug approvals but also supports the overall integrity of the research process.
The article outlines critical components of DMF filing, emphasizing the importance of thorough preparation and adherence to guidelines. From identifying the appropriate DMF type to following a structured submission process, each step plays a vital role in facilitating efficient regulatory evaluations. Additionally, maintaining and updating DMFs ensures ongoing compliance, allowing organizations to adapt to any changes in manufacturing practices or FDA regulations.
As the pharmaceutical industry continues to evolve, the significance of effective DMF management cannot be overstated. Engaging with regulatory experts and staying informed about industry standards empowers clinical research directors to optimize their DMF submissions. Ultimately, a well-prepared DMF not only protects sensitive information but also contributes to the advancement of drug development, reinforcing the importance of diligence and precision in this critical aspect of clinical research.
What is a Drug Master File (DMF)?
A DMF is a confidential submission to the FDA that provides comprehensive information about the facilities, procedures, and materials involved in the production, handling, packaging, and storage of human drug products.
Why is DMF filing important?
DMF filing is essential for safeguarding proprietary data while facilitating regulatory submissions, allowing companies to present vital details without disclosing sensitive information to the public. This confidentiality helps maintain competitive advantages in the pharmaceutical industry.
What are the key components of a DMF filing?
Key components of a DMF filing include information on manufacturing locations, quality assurance practices, and specific methods used in drug production.
How do DMFs impact regulatory processes?
By organizing DMFs effectively, companies can streamline their regulatory processes, ensuring compliance with FDA requirements. DMF filing is integral to Investigational New Drug Applications (INDs), New Drug Applications (NDAs), and Abbreviated New Drug Applications (ANDAs).
What is the significance of DMFs in clinical research?
The importance of DMF filing in clinical research continues to grow, as they protect proprietary data and enhance the efficiency of regulatory evaluations. Proper DMF filing is crucial for successful submissions.
What can happen if DMF submissions are improperly formatted or incomplete?
Improper formatting or incomplete submissions can result in significant delays in FDA reviews, emphasizing the necessity for thorough preparation in DMF filing.
When are DMFs primarily reviewed?
DMFs are primarily reviewed when referenced in applications, highlighting their strategic value in the regulatory landscape.
How can clinical research directors benefit from understanding DMFs?
By mastering the purpose and structure of DMFs, clinical research directors can establish a solid foundation for compliance and successful submissions in the regulatory process.