


The article delineates crucial steps for Medtech leaders to effectively navigate the EC certification process. It underscores the necessity of comprehending regulatory requirements, preparing thorough documentation, and engaging with Notified Bodies at the outset. By concentrating on meticulous preparation and strict adherence to EU regulations, which includes a comprehensive post-market surveillance plan, Medtech leaders can significantly enhance compliance and facilitate a more seamless market entry for their products.
Navigating the complex landscape of medical device certification presents a formidable challenge for Medtech leaders, particularly with the evolving regulations set to take effect in 2025. Understanding the EC certification process transcends mere regulatory compliance; it embodies a critical opportunity for companies to ensure their products meet stringent safety and efficacy standards, thereby enhancing public trust.
However, with approximately 30% of applications experiencing delays due to incomplete documentation and unclear communication with Notified Bodies, Medtech leaders must consider how to streamline their journey to compliance and market entry.
The EC approval procedure is critical for ensuring that medical instruments adhere to EU regulations. It commences with a comprehensive understanding of applicable directives, including the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). The process typically unfolds through the following stages:
Recent updates to the EU Medical Equipment Regulation in 2025 underscore the necessity for enhanced transparency and stringent evaluation processes, particularly concerning AI/ML-based medical products. Statistics indicate that approximately 70% of medical devices achieve EC approval on their first attempt, emphasizing the importance of meticulous preparation and adherence to regulatory standards. Successful examples within the Medtech sector illustrate the effectiveness of a well-structured credentialing strategy, reinforcing the need for compliance to bolster public trust and ensure patient safety.
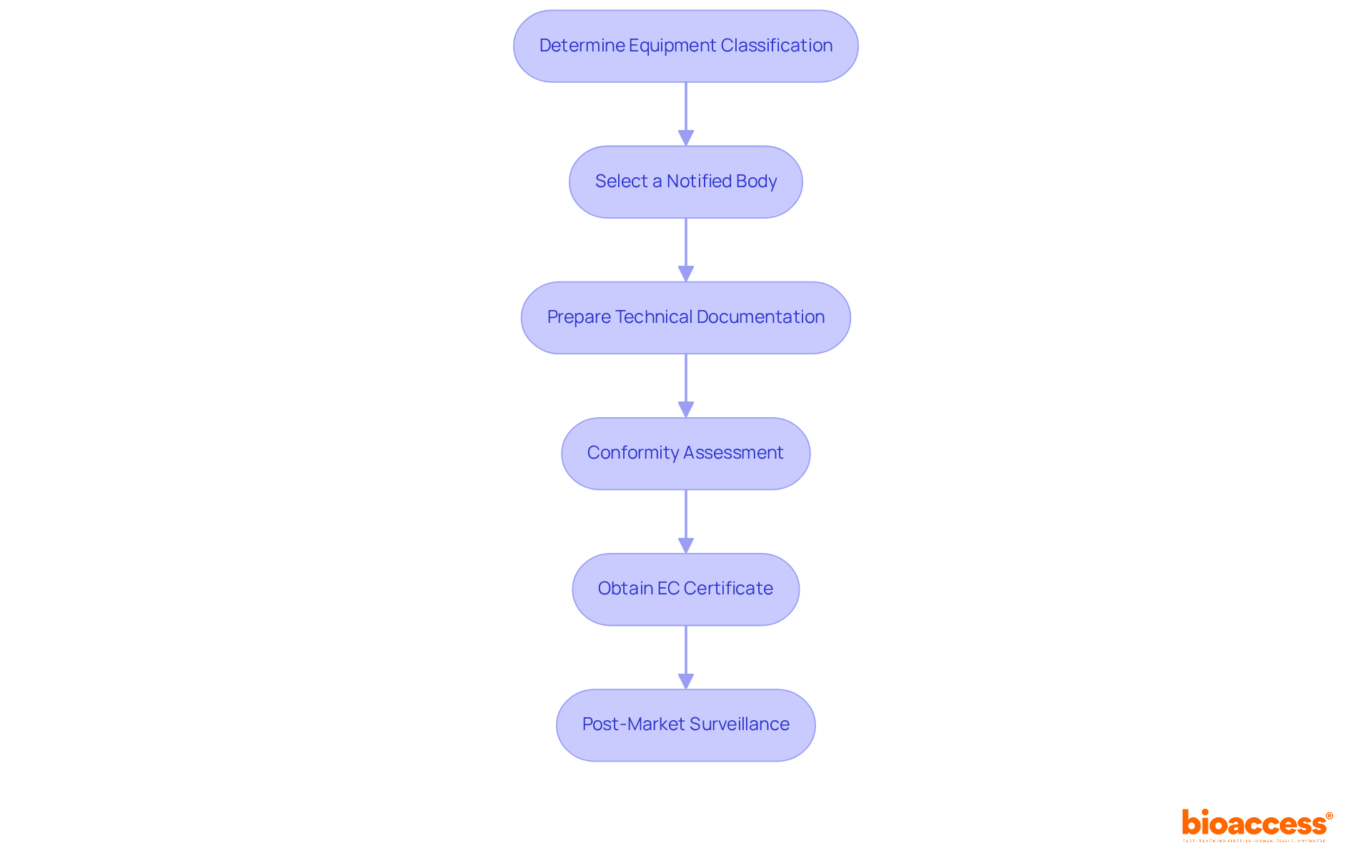
To successfully obtain EC certification, Medtech leaders must be aware of the following key requirements:
Technical Documentation: This encompasses essential components such as a comprehensive product description and intended use, comprehensive design and production methods, thorough risk management documentation—critical for ensuring safety and compliance—and clinical evaluation reports that support the efficacy and safety of the apparatus.
Quality Management System (QMS): Establishing a QMS compliant with ISO 13485 is vital. This standard outlines processes for quality assurance throughout the product lifecycle, ensuring that medical instruments consistently meet regulatory and safety standards. Notably, approximately 70% of Medtech companies have achieved ISO 13485 certification, underscoring its importance in the industry.
Declaration of Conformity: Prepare a Declaration of Conformity that explicitly states your equipment meets all relevant EU directives, a crucial step in demonstrating compliance.
Labeling and Instructions for Use: Ensure that all labeling adheres to EU regulations, including necessary warnings and instructions for safe use. Proper labeling is not only a regulatory requirement but also enhances user safety and product reliability.
Post-Market Surveillance Plan: Develop a robust plan for monitoring the device's performance after it enters the market. This includes establishing reporting mechanisms for adverse events, essential for ongoing safety assessments and regulatory compliance. Effective post-market surveillance is a key aspect of maintaining product quality and addressing any potential issues promptly.
By concentrating on these essential areas, Medtech leaders can maneuver through the complexities of EC cert approval more effectively, ensuring their products satisfy the stringent requirements of the European market.
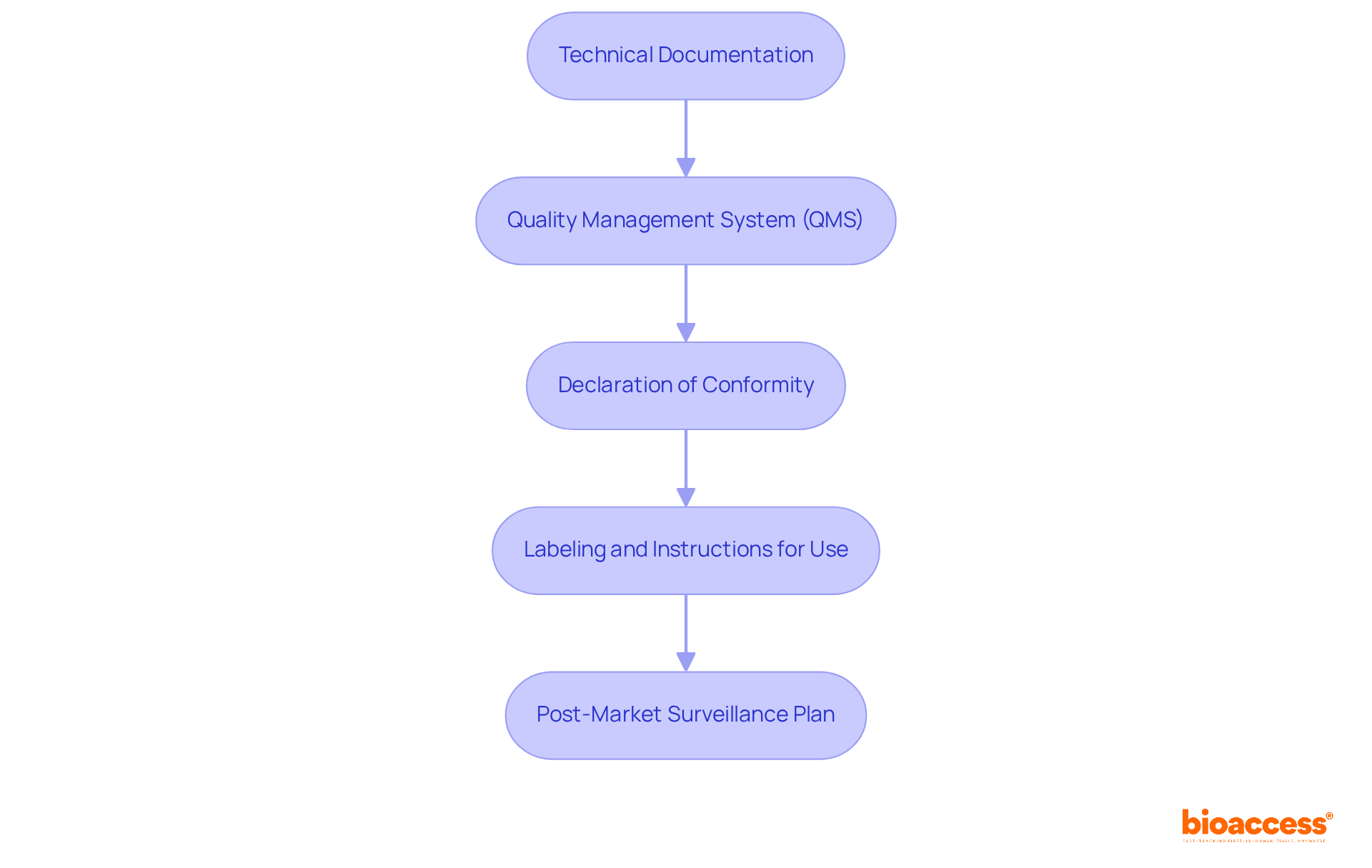
Preparing your application for EC certification involves several critical steps:
Gather Required Documentation: Collect all necessary documents, including technical files, risk assessments, and clinical evaluation reports. Ensuring completeness and accuracy is vital, as incomplete submissions can lead to delays.
Engage with a Recognized Body: Initiate communication with your chosen Recognized Body early in the process for the EC cert. Understanding their specific requirements and timelines can significantly streamline your application. Conformity assessment organizations often stress the significance of early involvement in achieving EC cert to clarify expectations and minimize the risk of non-compliance.
Complete the Application Form: Fill out the application form given by the Authorized Body meticulously. Accurate and complete information is crucial for the EC cert approval process, as any discrepancies can hinder it.
Submit Technical Documentation: Provide your technical documentation along with the application form, ensuring it complies with the Body's standards. This documentation should demonstrate compliance with the relevant EU directives and include all necessary evidence of safety and performance for the EC cert.
Prepare for Audits: Be ready for potential audits or inspections by the Authorized Body. This may include site visits and thorough document reviews. Getting acquainted with the audit procedures related to the EC cert can help reduce any surprises and enable a smoother evaluation.
Follow Up: Keep open lines of communication with the Alerted Body throughout the review process. Quickly responding to any inquiries or extra needs can aid in speeding up the EC cert approval schedule, which can differ greatly depending on device classification and the assessment body's capacity.
Statistics indicate that early engagement with Notified Bodies can reduce approval timelines by up to 30%, highlighting the value of proactive communication. Successful examples from the Medtech sector show that companies that prioritize these steps often achieve faster market entry and enhanced compliance.
Navigating the EC cert accreditation process presents several challenges that can impede progress. To effectively troubleshoot these issues, consider the following strategies:
Ensure Complete Documentation: Thoroughly review all required documents for accuracy and completeness prior to submission. Incomplete documentation can significantly delay the ec cert timeline, making a meticulous approach essential. Experts note that the consequences of misinformation can exacerbate these delays, highlighting the importance of verifying all information to obtain an ec cert.
Foster Clear Communication with Notified Bodies: Establish and maintain open lines of communication with your Notified Body. Swiftly address any uncertainties regarding requirements or procedures to avoid misunderstandings that could lead to setbacks. Steven Miller emphasizes the responsibility of individuals to seek reliable sources, which is vital in this context.
Stay Informed on Regulatory Changes: Regularly track updates to EU regulations that may affect your approval journey. Engaging with regulatory resources or consulting legal experts can help you adapt to changes swiftly. The dynamic nature of regulations means that staying informed is not just beneficial but necessary.
Allocate Adequate Resources: Ensure that sufficient time and personnel are dedicated to managing the accreditation process. If internal resources are limited, consider hiring external consultants with expertise in EC cert to streamline efforts. This approach can mitigate the risks associated with resource constraints.
Implement a Strong Post-Market Surveillance Plan: Develop a comprehensive post-market surveillance strategy to identify and address potential issues that may arise after certification. This proactive approach not only enhances compliance but also mitigates the risk of regulatory complications down the line. As highlighted in case studies, effective post-market strategies can significantly reduce the likelihood of facing issues related to incomplete documentation.

Navigating the EC certification process is a vital undertaking for Medtech leaders, offering a pathway not only to compliance but also to building trust with the public. Mastering this process requires a thorough understanding of regulations, meticulous preparation, and proactive engagement with Notified Bodies. Successfully achieving EC certification ensures that medical devices meet the highest safety and efficacy standards, which is essential in today’s competitive healthcare landscape.
Key elements of the certification journey include:
Each step, from selecting a Notified Body to implementing a robust post-market surveillance plan, plays a crucial role in mitigating risks and overcoming common challenges. By adhering to these guidelines, Medtech leaders can enhance their chances of a smooth certification process and expedite their time to market.
Ultimately, the significance of EC certification extends beyond regulatory compliance; it represents a commitment to patient safety and product reliability. Medtech leaders are encouraged to embrace these best practices, remain vigilant about regulatory changes, and foster open communication with their Notified Bodies. Taking these proactive steps not only streamlines the certification process but also reinforces the integrity of the Medtech industry as a whole, paving the way for innovation and trust in medical devices.
What is the EC certification process for medical instruments?
The EC certification process ensures that medical instruments comply with EU regulations, beginning with understanding applicable directives like the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR).
What are the main stages of the EC certification process?
The main stages include determining equipment classification, selecting a Notified Body, preparing technical documentation, undergoing a conformity assessment, obtaining the EC certificate, and implementing post-market surveillance.
How is equipment classification determined in the EC certification process?
Equipment classification is determined according to EU regulations, which dictates the level of scrutiny the product will face during the certification procedure.
What is a Notified Body, and why is it important?
A Notified Body (NB) is an organization authorized to evaluate a product's conformity. It is important to select an NB with the necessary expertise in the product's category to ensure proper evaluation.
What kind of documentation is required for the EC certification process?
Required documentation includes design specifications, risk assessments, and clinical evaluations, which are essential for demonstrating compliance with EU regulations.
What does the conformity assessment process involve?
The conformity assessment process involves audits and testing conducted by the selected Notified Body to verify that the product complies with EU standards.
What happens after obtaining the EC certificate?
After obtaining the EC certificate, you can affix the CE mark to your product, which is crucial for market access.
What is post-market surveillance, and why is it necessary?
Post-market surveillance is a plan implemented to continuously monitor the product's performance and report any issues to relevant authorities, ensuring ongoing compliance and safety.
What recent updates have been made to the EU Medical Equipment Regulation?
Recent updates in 2025 emphasize enhanced transparency and stricter evaluation processes, particularly for AI/ML-based medical products.
What is the success rate for obtaining EC approval on the first attempt?
Approximately 70% of medical devices achieve EC approval on their first attempt, highlighting the importance of meticulous preparation and adherence to regulatory standards.