


Navigating the complexities of clinical research in Bosnia demands a thorough understanding of its ethical and regulatory frameworks. Researchers must grapple with a landscape shaped by both local laws and international standards, facing the dual challenge of ensuring compliance while upholding the integrity of their studies. As Bosnia strives to align its practices with European Union directives, a pressing question emerges: how can researchers effectively harmonize these regulations to cultivate a trustworthy and efficient research environment?
This article explores essential strategies and opportunities for mastering ethics and regulatory harmonization in Bosnia. By empowering researchers to enhance their practices, we aim to contribute meaningfully to the evolving medical landscape. Through a blend of authoritative insights and practical guidance, we will delve into the critical aspects of navigating this intricate terrain.
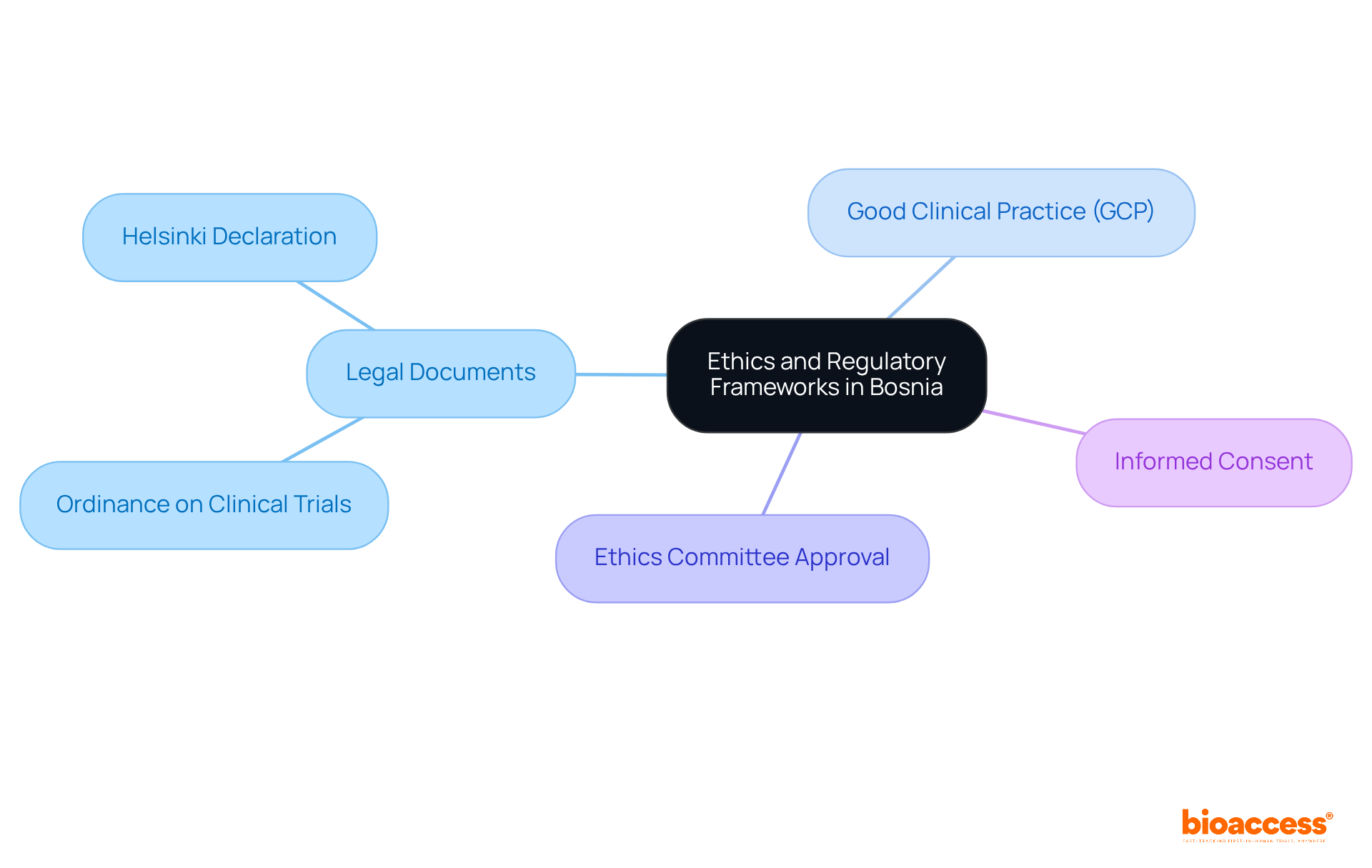
To effectively conduct medical research in Bosnia, it is crucial to understand the ethics and regulatory harmonization in Bosnia. The primary legal documents include the Ordinance on Clinical Trials on Medicinal Products and Medical Devices, which details the procedures for obtaining approvals from the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina (ALMBiH). Additionally, adherence to the Helsinki Declaration is vital, ensuring that all research studies respect the rights and well-being of participants.
Key components of the regulatory framework include:
bioaccess offers comprehensive trial management services that align with these guidelines, including feasibility studies, site selection, review processes, trial setup, import permits, project management, and reporting. This holistic approach not only ensures compliance with ethics and regulatory harmonization in Bosnia but also enhances the credibility and integrity of the research conducted.
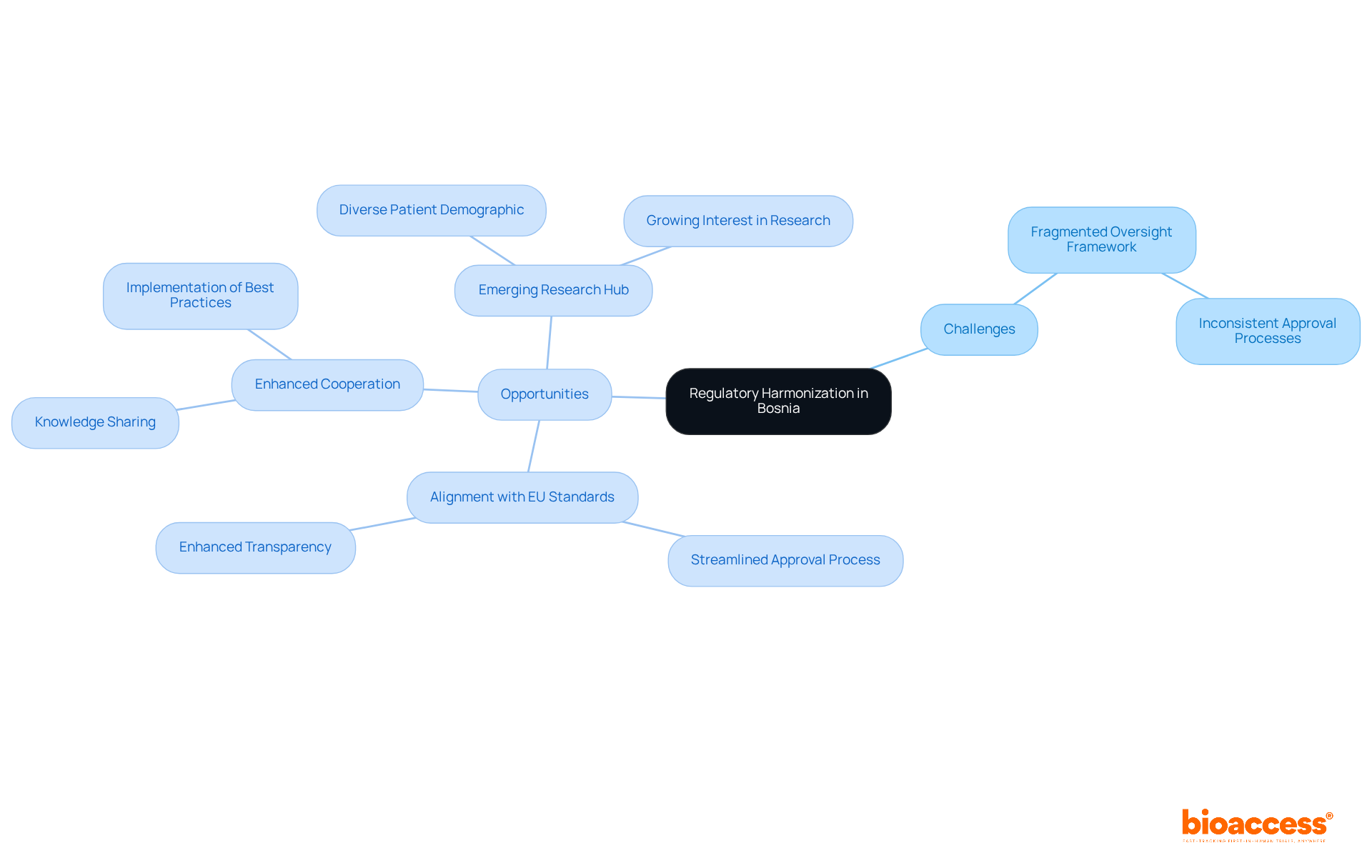
The oversight landscape in Bosnia presents both challenges and opportunities for clinical research, especially regarding ethics and regulatory harmonization in Bosnia. A significant hurdle is the fragmented oversight framework, leading to inconsistent approval processes across various regions. While some ethics committees expedite approvals, others may impose prolonged review timelines, affecting the ethics and regulatory harmonization in Bosnia by causing delays in the initiation of studies.
Opportunities for Regulatory Harmonization:
By understanding these dynamics, researchers can more effectively navigate the regulatory framework and seize the opportunities available, ultimately enhancing ethics and regulatory harmonization in Bosnia.
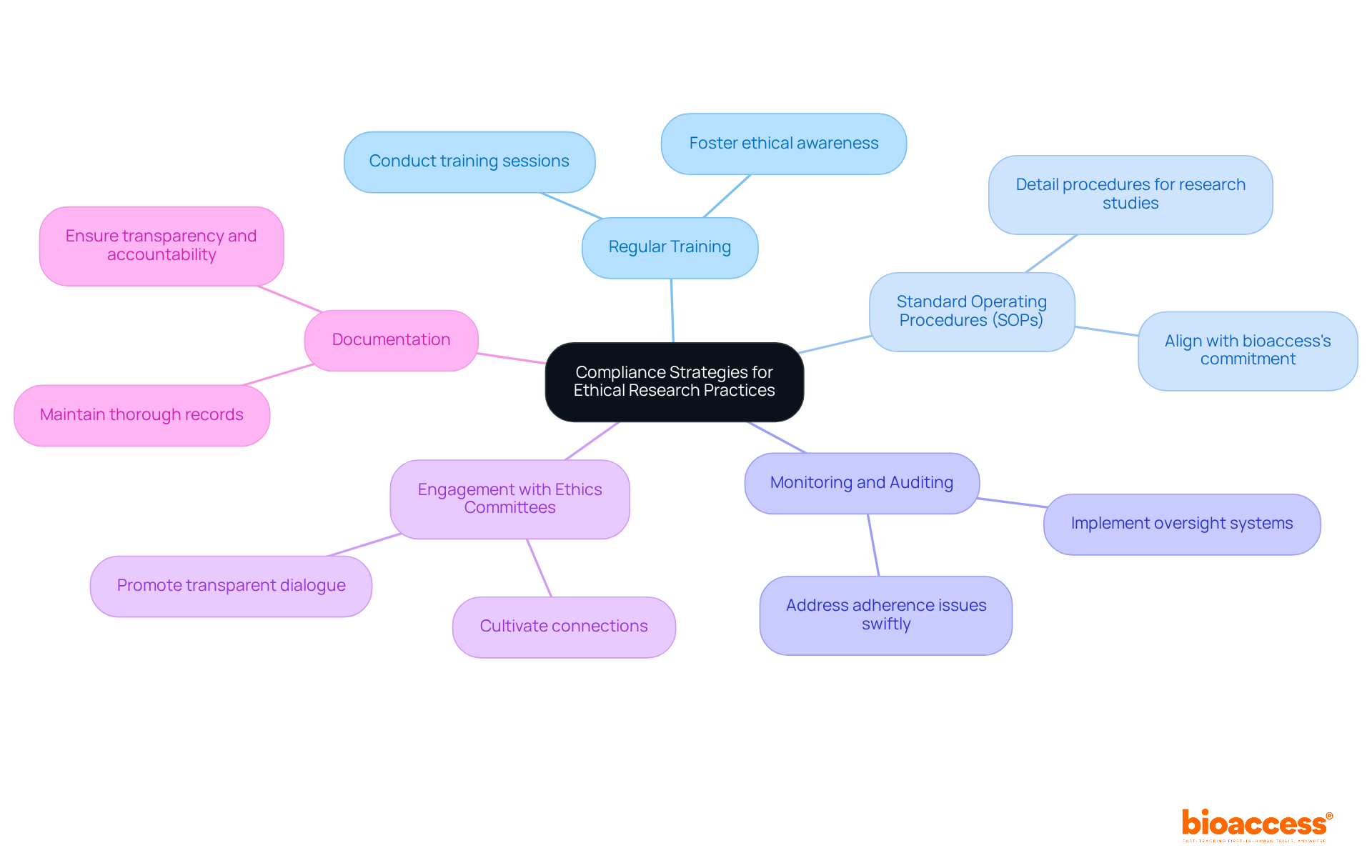
To ensure ethics and regulatory harmonization in Bosnia, it is essential to implement strong adherence strategies for ethical research practices. Here are key strategies to consider:
By implementing these strategies, researchers can enhance the ethical conduct of their trials and build trust with participants and stakeholders.
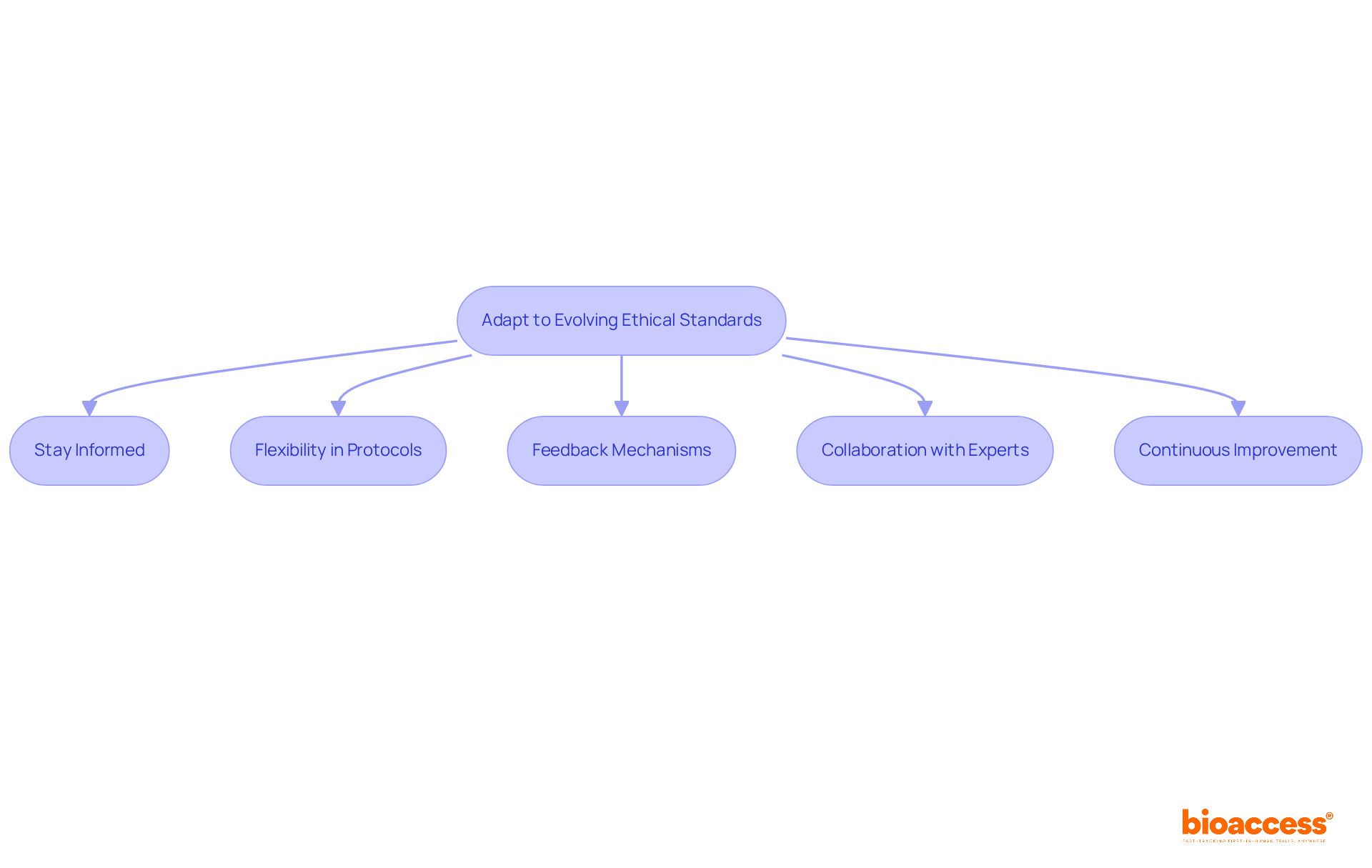
The landscape of medical research is in a constant state of flux, with new ethical guidelines, including ethics and regulatory harmonization in Bosnia, and compliance changes emerging regularly. To navigate this evolving terrain effectively, researchers must take proactive steps:
By actively adapting to these changes, researchers can significantly enhance the ethical integrity of their studies and ensure compliance with the ethics and regulatory harmonization in Bosnia.
Understanding the complexities of ethics and regulatory harmonization in Bosnia is crucial for successful clinical research. The legal frameworks, including the Ordinance on Clinical Trials and adherence to the Helsinki Declaration, establish ethical standards that protect participants and ensure credible data. By recognizing these foundational elements, researchers can navigate the Bosnian regulatory landscape more effectively.
This article highlights critical components such as:
It also addresses the challenges posed by a fragmented oversight system while presenting opportunities for alignment with EU standards, enhanced cooperation, and the potential for Bosnia to emerge as a research hub. Implementing compliance strategies, such as regular training and monitoring, further solidifies the ethical foundation necessary for successful trials.
Ultimately, the commitment to ethical research practices transcends mere regulatory adherence; it fosters trust and integrity within the clinical research community. As Bosnia continues to evolve its regulatory frameworks, researchers are encouraged to stay informed, adapt to changes, and collaborate with experts to ensure that ethical standards remain at the forefront of their work. Embracing these principles will not only enhance the quality of research but also contribute to the overall advancement of medical science in the region.
What is essential for conducting medical research in Bosnia?
Understanding the ethics and regulatory harmonization in Bosnia is crucial for conducting medical research effectively.
What are the primary legal documents governing medical research in Bosnia?
The primary legal document is the Ordinance on Clinical Trials on Medicinal Products and Medical Devices, which outlines the procedures for obtaining approvals from the Agency for Medicinal Products and Medical Devices of Bosnia and Herzegovina (ALMBiH).
Why is adherence to the Helsinki Declaration important in medical research?
Adherence to the Helsinki Declaration is vital to ensure that all research studies respect the rights and well-being of participants.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is an international quality standard that guarantees clinical trials are conducted ethically and that the data generated is credible.
What is required before conducting a medical study in Bosnia?
All medical studies must secure approval from a local ethics committee, which evaluates the ethical implications of the proposed research.
What is the importance of informed consent in medical research?
Informed consent is crucial as participants must be thoroughly informed about the study's purpose, procedures, risks, and benefits before agreeing to participate.
What services does bioaccess offer to support compliance with ethical and regulatory guidelines?
bioaccess offers comprehensive trial management services, including feasibility studies, site selection, review processes, trial setup, import permits, project management, and reporting, ensuring compliance with ethics and regulatory harmonization in Bosnia.