


The essential steps for achieving clinical research success in mastering FDA approval are:
Understanding each stage of the approval process—from preclinical to post-market surveillance—is crucial. Utilizing expert resources significantly enhances the likelihood of successful product commercialization. This comprehensive approach not only fosters a deeper understanding of the Medtech landscape but also addresses key challenges faced by researchers.
Navigating the intricate landscape of FDA approval is essential for any medical device or pharmaceutical company aiming to bring innovative products to market. This comprehensive process, which spans from preclinical research to post-market surveillance, is critical for ensuring the safety and efficacy of new therapies. However, the journey is fraught with challenges, prompting stakeholders to consider: what strategies can they employ to enhance their chances of success in this complex approval maze?
By exploring the key stages and best practices of the FDA approval process, we uncover not only the importance of meticulous preparation but also the potential pitfalls that can derail even the most promising research endeavors.
The FDA approval process is a vital gateway for medical devices and pharmaceuticals seeking market entry. This multifaceted journey encompasses several key stages:
Each stage is essential for ensuring compliance with regulatory standards and facilitating the successful commercialization of innovative products. A primary focus of this procedure is the thorough assessment of safety and efficacy through research studies that collect vital data necessary for FDA approval.
Adhering to Good Practice in Research (GCP) guidelines is not merely a regulatory necessity; it is a fundamental aspect of effective research. This foundational knowledge equips stakeholders to navigate the complexities of obtaining approval adeptly, ultimately enhancing the likelihood of successful product launches in a competitive market.
At bioaccess®, we provide extensive clinical study management services, including:
Our expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies positions us as a trusted partner for U.S. medical device companies aiming to advance their products in Latin America.
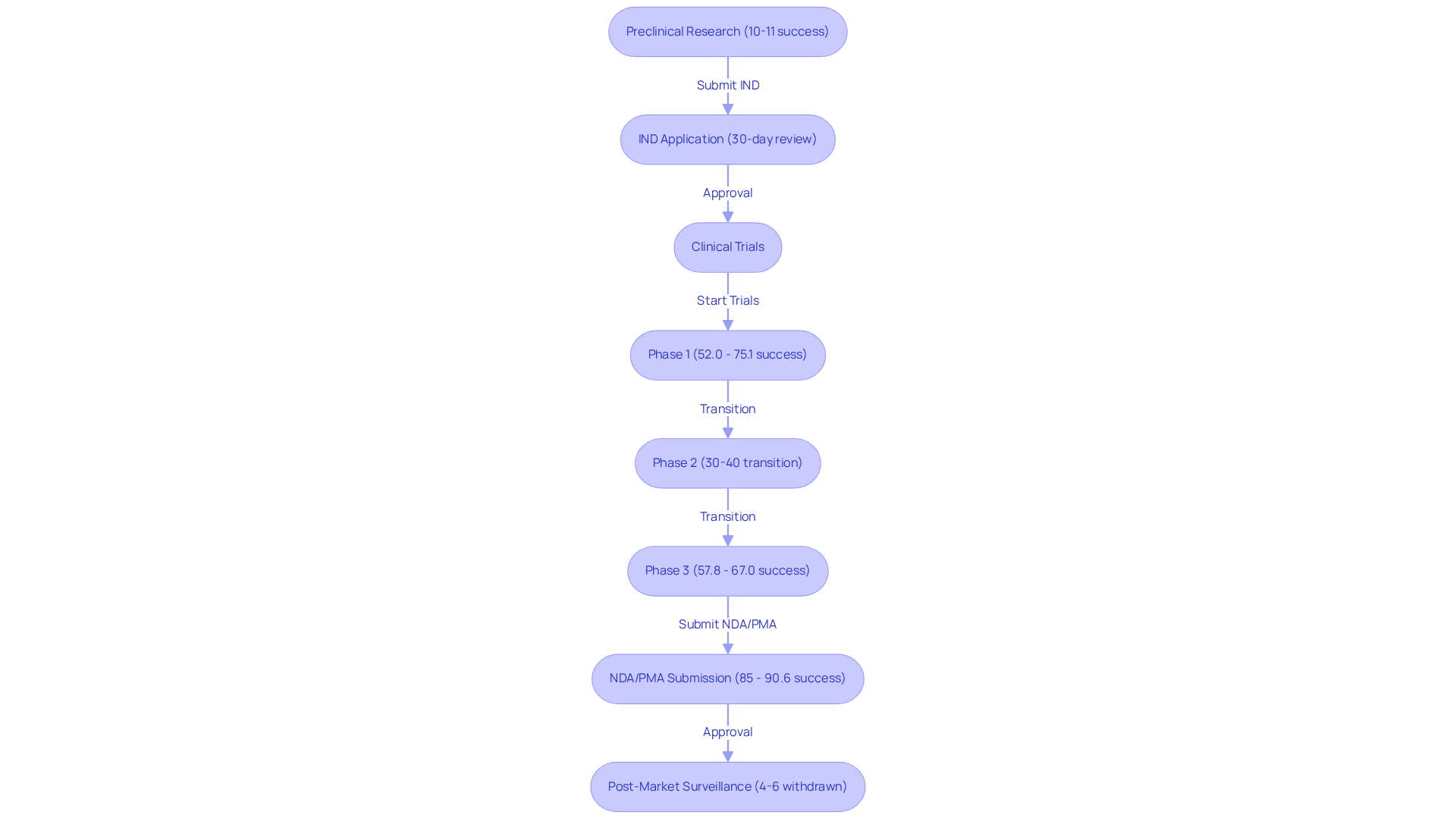
The FDA approval process encompasses several critical stages that ensure the safety and efficacy of new drugs:
Preclinical Research: This foundational phase involves rigorous laboratory and animal testing to evaluate safety and biological activity. It is crucial for assessing whether a product is appropriate for human testing, with roughly 10-11% of drugs advancing from this stage to Phase 1 evaluations. Thorough management services for studies, such as feasibility assessments, play an essential role in evaluating the viability of the research location and principal investigator (PI) during this phase.
Before starting human studies, sponsors are required to submit an IND application to obtain FDA approval. This application contains extensive information from preclinical research and details the suggested research protocols. The FDA approval process usually evaluates IND applications within a 30-day timeframe, and successful submissions are essential for progressing to research studies. Bioaccess provides review and feedback on study documents to ensure compliance with country requirements, which is essential for a successful IND application.
Clinical Trials: This phase is divided into three distinct stages:
After successful studies, an NDA or PMA is submitted for FDA approval, which includes all study data. The success rate for NDAs in obtaining FDA approval is notably high, ranging from 85% to 90.6%, underscoring the importance of thorough preparation. Bioaccess assists in the preparation of these applications, ensuring all necessary data is included.
Post-Market Surveillance: After a product receives approval, the FDA continues to monitor its safety and effectiveness through post-marketing studies and adverse event reporting. This ongoing evaluation is vital for ensuring long-term patient safety, as approximately 4%-6% of drugs with FDA approval may be withdrawn from the market due to safety concerns post-approval. Bioaccess provides reporting services to track study status and adverse events, contributing to effective post-market surveillance.
Comprehending these phases, especially the importance of preclinical studies and the IND application, is crucial for research directors striving for success in drug development. By utilizing extensive trial management services, including feasibility evaluations, site selection, compliance assessments, trial organization, import permits, project oversight, and reporting, sponsors can navigate the complexities of FDA approval more effectively.
Navigating the pathway to FDA approval necessitates a strategic approach to overcome inherent challenges. Here are key strategies to enhance your chances of success:
By applying these strategies, clinical research teams can effectively navigate the intricacies of obtaining FDA approval, ultimately expediting the route to market for innovative therapies.

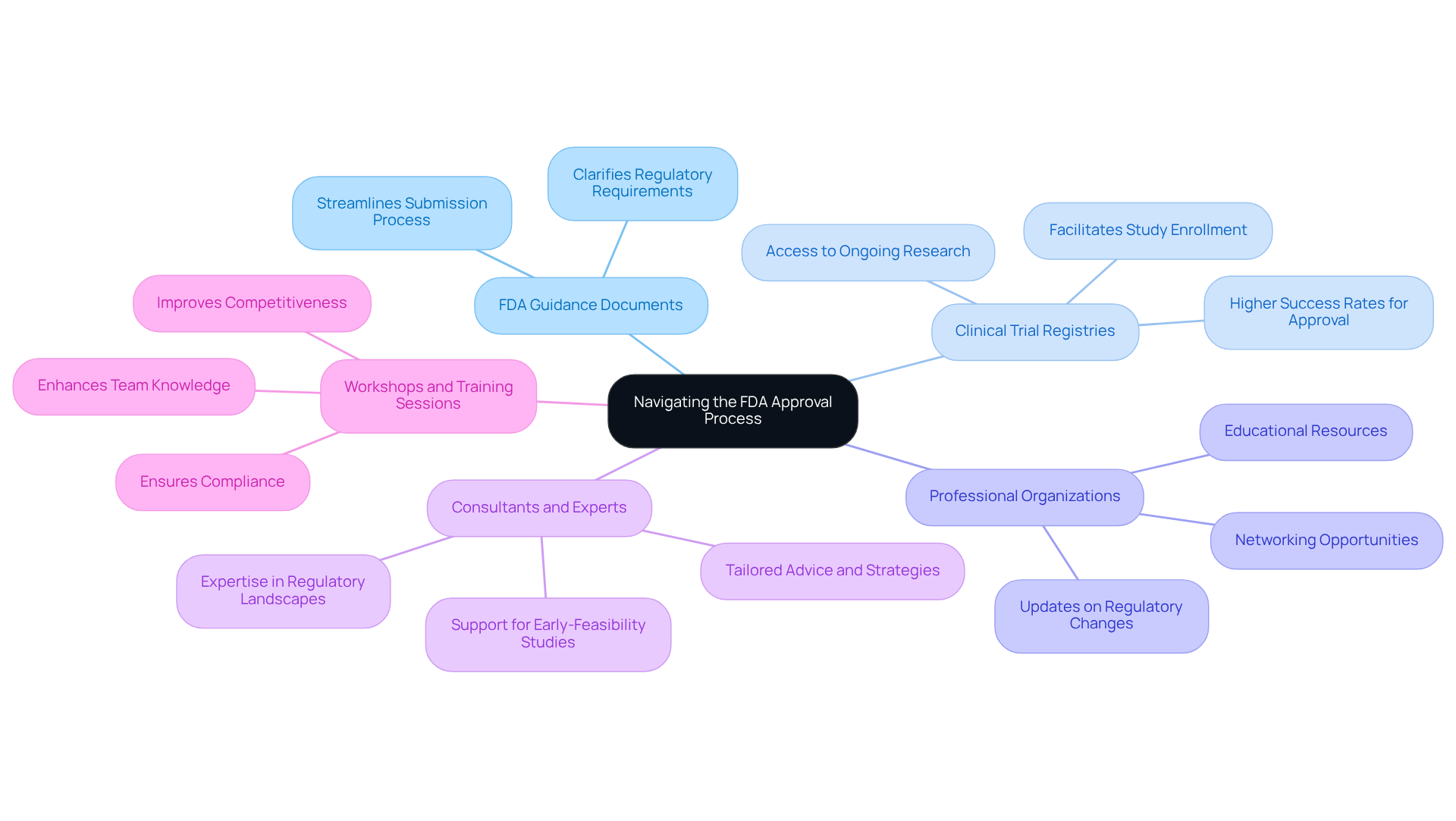
To successfully navigate the FDA approval process, leveraging the following resources is essential for enhancing your chances of success:
FDA Guidance Documents: The FDA offers a comprehensive array of guidance documents that detail expectations for various submission types. Familiarizing yourself with these documents can clarify regulatory requirements and streamline your submission process.
Clinical Trial Registries: Utilizing platforms like ClinicalTrials.gov is crucial. These registries facilitate the enrollment of your studies and provide access to information on ongoing research, which can significantly inform and enhance your research design. Notably, studies registered on these platforms often demonstrate higher success rates for FDA approval.
Professional Organizations: Joining organizations such as the Regulatory Affairs Professionals Society (RAPS) presents valuable networking opportunities, educational resources, and timely updates on regulatory changes that may impact your submissions.
Consultants and Experts: Engaging regulatory consultants with specialized expertise in FDA submissions can yield tailored advice and strategies, drawing from their extensive experience to navigate complex regulatory landscapes effectively. bioaccess® stands out as a reputable CRO and consulting ally for U.S. medical device firms, offering comprehensive research management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting. Their proficiency in overseeing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH) ensures that your experiments are conducted effectively and in accordance with regulatory standards.
Participating in workshops and training sessions focused on FDA approval and clinical trial management can significantly enhance your team's knowledge and skills, ensuring compliance and competitiveness in the evolving regulatory environment.
Navigating the FDA approval process is an essential undertaking for medical device and pharmaceutical companies striving to introduce innovative products to the market. This intricate journey encompasses vital stages, including:
All designed to guarantee that new therapies are both safe and effective. A comprehensive understanding of this process not only bolsters compliance with regulatory standards but also significantly enhances the probability of successful product launches in a competitive landscape.
Throughout this article, critical insights have been shared regarding the significance of:
Furthermore, the focus on data quality and strategic planning for post-market requirements underscores the complexities involved in obtaining FDA approval. By leveraging regulatory resources, such as guidance documents and professional organizations, stakeholders can streamline their submissions and improve their chances of success.
Ultimately, the journey through the FDA approval process transcends mere compliance with regulatory requirements; it is fundamentally about ensuring patient safety and fostering innovation within healthcare. Adopting strategic approaches and utilizing available resources can markedly facilitate the pathway to market entry. For those engaged in clinical research, the dedication to comprehending and navigating this critical process is paramount, as it serves as the key to transforming groundbreaking ideas into life-saving therapies.
What is the FDA approval process?
The FDA approval process is a crucial pathway for medical devices and pharmaceuticals seeking to enter the market, involving several key stages: preclinical research, medical studies, and post-market surveillance.
Why is the FDA approval process important?
The FDA approval process is essential for ensuring compliance with regulatory standards and for the successful commercialization of innovative medical products. It focuses on assessing safety and efficacy through research studies that gather necessary data for FDA approval.
What are the key stages of the FDA approval process?
The key stages of the FDA approval process include preclinical research, medical studies, and post-market surveillance.
What guidelines must be followed during the FDA approval process?
Adhering to Good Practice in Research (GCP) guidelines is essential during the FDA approval process, as it is a regulatory necessity and a critical aspect of effective research.
What services does bioaccess® provide related to clinical study management?
bioaccess® offers extensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting.
What types of studies does bioaccess® manage?
bioaccess® manages Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies.
Who can benefit from the services provided by bioaccess®?
U.S. medical device companies aiming to advance their products in Latin America can benefit from the services provided by bioaccess®.