


The article centers on mastering the FDA approval process for medical devices, outlining essential steps and strategies that are crucial for success. It underscores the significance of comprehending device classifications and regulatory pathways, such as 510(k) and PMA, alongside post-approval requirements. Supported by compelling statistics and insights from experts, this article enhances the likelihood of successful market entry.
Navigating the complex landscape of FDA approval for medical devices presents a formidable challenge for manufacturers and innovators. With stringent regulations and multiple pathways to consider, grasping the nuances of the approval process is vital for achieving success. This article serves as a comprehensive step-by-step guide, demystifying the FDA device approval process while also highlighting best practices and strategies designed to enhance the likelihood of a successful submission.
How can one effectively maneuver through the intricacies of FDA regulations while ensuring compliance and market readiness?
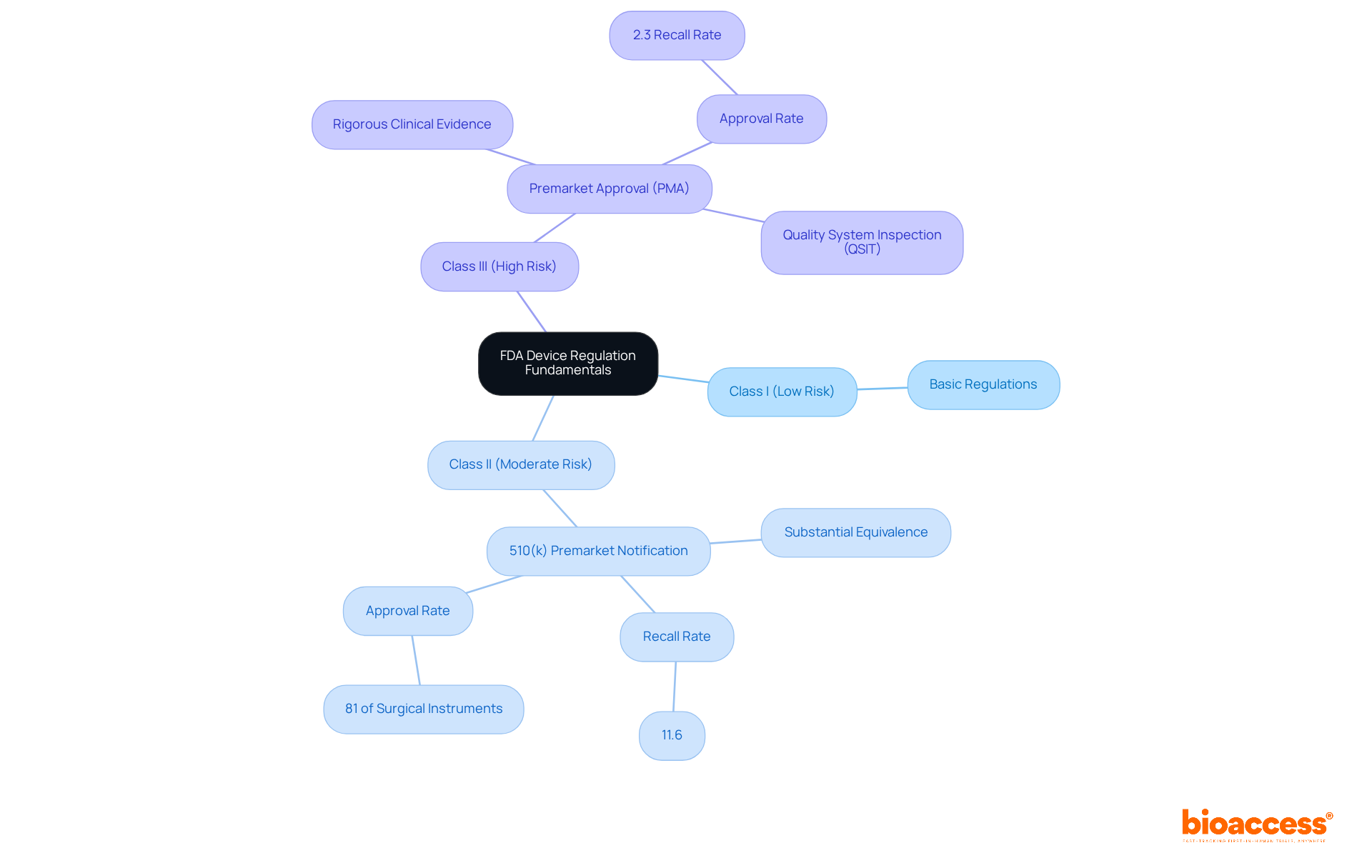
To master the process of FDA approval devices for medical equipment, it is essential to familiarize yourself with the key regulations governing medical products in the United States. The FDA categorizes equipment into three classifications based on risk:
Each class has distinct regulatory requirements. For instance, Class I products typically require basic regulations, while Class II products often necessitate a 510(k) premarket notification to demonstrate substantial equivalence to a legally marketed item. In contrast, Class III products demand a more stringent Premarket Approval (PMA) process, which involves comprehensive clinical data to establish safety and efficacy. Understanding these classifications is crucial, as they dictate the pathway for approval.
Additionally, it is important to familiarize yourself with the FDA's guidance documents and regulations concerning FDA approval devices, which provide detailed information on compliance and best practices. Recent statistics reveal that 81% of surgical instruments were approved via the 510(k) pathway, which had a recall rate of 11.6%, whereas items approved through PMA experienced a significantly lower recall rate of 2.3%. Furthermore, 510(k) product recalls are 5.32 times more likely than PMA product recalls, underscoring the necessity for rigorous evaluation of high-risk items. The average time to receive a decision on FDA 510(k) applications is approximately 147 days, highlighting the efficiency of this pathway.
With experts like Ana Criado, Director of Regulatory Affairs at bioaccess, who possesses extensive experience in regulatory affairs and clinical trials, navigating these complexities becomes more manageable. Bioaccess also offers comprehensive clinical trial management services, including:
This ensures a streamlined process for your medical product approval.
The FDA offers multiple routes for FDA approval of devices, each tailored for specific types and associated risks. The primary pathways include:
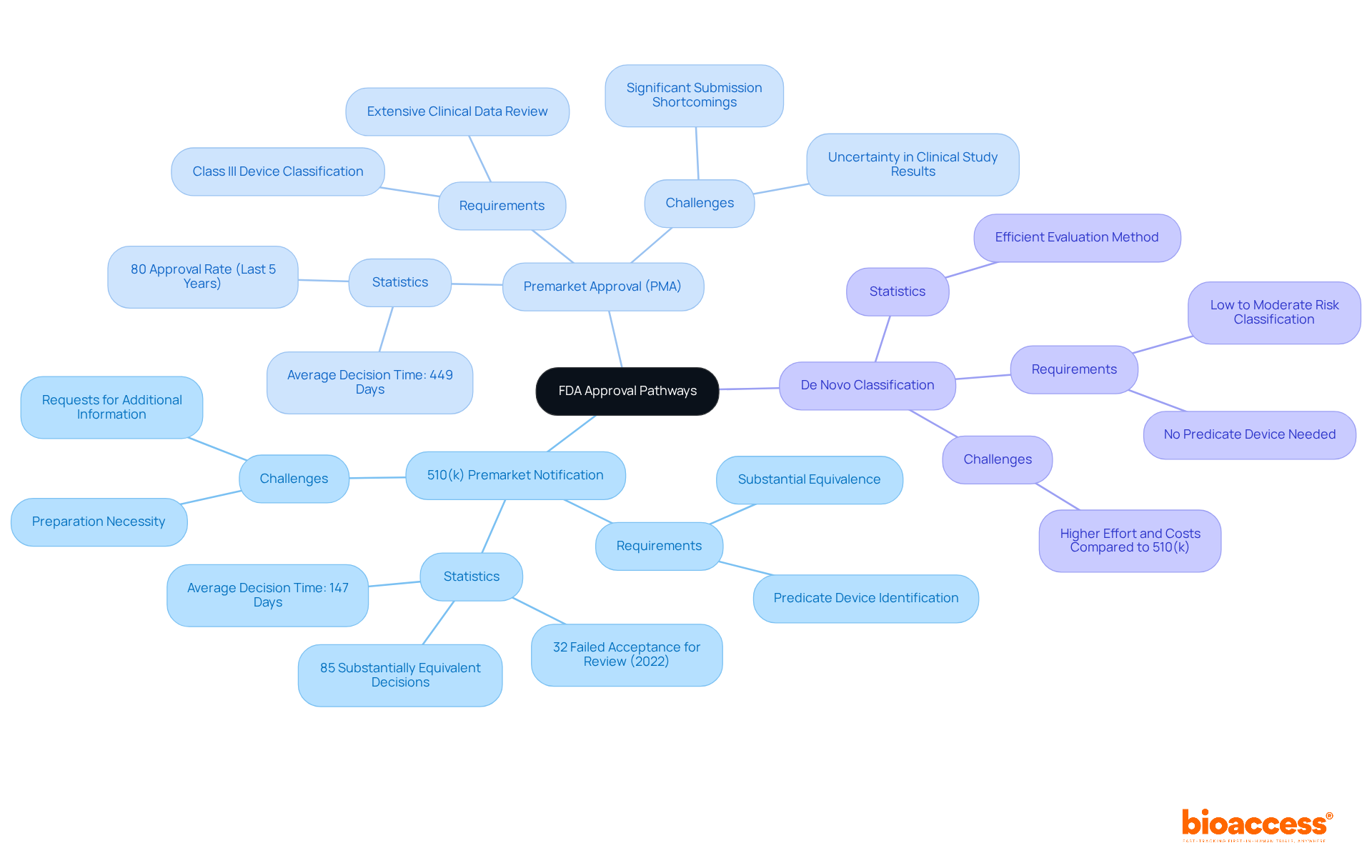
510(k) Premarket Notification: This pathway is utilized for products demonstrating substantial equivalence to an already marketed item. A submission is typically required at least 90 days before marketing. Manufacturers must identify a predicate item and show that their product is as safe and effective as the existing one. Recent statistics reveal that 85 percent of 510(k) applications received a Substantially Equivalent decision; however, 32 percent failed the acceptance for review check in 2022, underscoring the necessity of thorough preparation.
Premarket Approval (PMA): The most stringent route, required for Class III instruments, necessitates an extensive review of clinical data to confirm the device's safety and effectiveness. The PMA process can be time-consuming, with original applications averaging 449 days for a decision, while Panel Track entries average 327 days. Industry leaders express concerns regarding the PMA procedure, citing challenges such as uncertainty about clinical study results and the potential for significant shortcomings in submissions.
De Novo Classification: This pathway accommodates new products classified as low to moderate risk that lack a predicate. It offers an efficient evaluation method, potentially facilitating a quicker market entry for innovative products. Despite its advantages, there is a common perception that the De Novo procedure requires more effort and incurs greater costs than the 510(k) pathway.
Understanding these pathways is crucial for aligning your development strategy with the pertinent regulatory requirements, ultimately fostering a more seamless approval process.
To prepare a successful FDA submission, it is imperative to follow these essential steps:
Gather Necessary Documentation: Compile all required documents, including device descriptions, intended use statements, labeling, and clinical data. Ensure that all information is accurate and consistent across documents, as discrepancies can lead to delays or rejections. High-quality, precise entries are essential. Industry specialists, such as Gary Saner, highlight that the integrity of entries can significantly affect patient safety and regulatory compliance.
Develop a Regulatory Strategy: Create a clear regulatory roadmap before drafting your proposal. This roadmap should outline your approach, including the chosen pathway (510(k), PMA, or De Novo) and the rationale behind it. The FDA has recently drafted guidance for a quicker approval path as an alternative to the 510(k), which could be beneficial for your strategy. A well-defined strategy can significantly enhance your entry's success rate.
Engage with the FDA Early: Arrange a pre-application meeting with the FDA to discuss your device and application strategy. This proactive involvement can offer valuable feedback, clarify uncertainties, and align expectations, ultimately leading to a smoother completion. Case studies have shown that effective pre-submission meetings can lead to better outcomes and higher approval rates.
Follow Guidelines for Submitting: Adhere strictly to the FDA's criteria, including formatting and content requirements. Utilize resources like the FDA's eSTAR program for electronic entries, which can streamline the process and enhance the quality of your entry. It is noteworthy that 74% of approvals in 2024 cleared on the first review, underscoring the importance of following best practices.
Review and Revise: Conduct a thorough evaluation of all documents prior to sending them to ensure completeness and compliance. Engaging a regulatory specialist in this review can assist in recognizing potential issues and improving the overall quality of your proposal. Continual oversight of UDI entries is essential for compliance, necessitating regular updates and precision in product information.
In addition to these steps, consider leveraging comprehensive clinical trial management services, such as those offered by bioaccess. These services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. By integrating these services into your submission process, you can enhance the quality of your FDA submission and increase the likelihood of a successful approval.

Once your device receives FDA approval, several post-approval requirements and market access strategies must be considered:
Post-Market Surveillance: Following approval, monitoring the performance of the product in the market is essential. This includes timely reporting of any adverse events, with nearly a third of such reports submitted late, often exceeding six months after manufacturers are notified. Conducting post-market studies may also be necessary to ensure ongoing safety and efficacy.
Quality Management System (QMS): A robust QMS is vital for maintaining compliance with FDA regulations. Regular audits and updates to quality protocols are essential, particularly considering the FDA approval devices in relation to the recent amendments to the Quality System Regulation (QSR) to better align with ISO 13485:2016. Companies utilizing industry-specific quality management tools are twice as likely to meet their quality goals compared to those relying on general-purpose solutions.
Market Access Strategies: Developing a comprehensive market access strategy is crucial. This should encompass pricing, reimbursement, and distribution plans. Engaging with payers early in the process is essential to understand their requirements and ensure that your FDA approval devices are adequately covered, facilitating smoother market entry. bioaccess® offers strategic reimbursement and health economics solutions tailored for Latin America, helping to navigate complex reimbursement landscapes and optimize pricing strategies for innovative therapies.
Regulatory updates: Staying informed about changes in regulations regarding FDA approval devices and guidance is imperative. Regularly reviewing and updating compliance strategies to align with new requirements will help mitigate risks associated with regulatory non-compliance. With expertise in regulatory affairs, bioaccess® can assist in implementing regulatory strategies to enhance your product's market entry.
Stakeholder Engagement: Establishing connections with important stakeholders, such as healthcare providers, payers, and regulatory bodies, is crucial for enabling market entry and securing continuous support for your product. A collaborative approach can significantly enhance the likelihood of success in the competitive healthcare landscape. Monica Mora, Chief Operating Officer at bioaccess®, specializes in operations and logistics, ensuring that your engagement strategies are effective and aligned with market needs.
By effectively navigating these post-approval requirements and implementing strategic market access plans, including utilizing bioaccess's toolkit of interactive pricing calculators and reimbursement rate databases, you can significantly enhance the success of your medical device in the evolving healthcare market.
Mastering the FDA approval process for medical devices is a critical endeavor for manufacturers aiming to bring innovative healthcare solutions to market. Understanding the essential classifications, pathways, and best practices outlined in this guide enables stakeholders to navigate the complexities of regulatory requirements more effectively. The journey begins with a solid grasp of device classifications and the associated regulatory frameworks, which set the foundation for successful submissions.
Key insights include:
These are pivotal steps that can significantly increase the likelihood of approval. Furthermore, post-approval monitoring and strategic market access planning are vital for ensuring long-term success and compliance in the competitive healthcare landscape.
In conclusion, the information presented in this guide serves as a roadmap for navigating the FDA approval process. By implementing the strategies discussed, manufacturers can enhance their chances of not only achieving FDA approval but also ensuring their devices effectively meet market needs. Embracing these best practices is essential for fostering innovation and delivering safe, effective medical devices that can ultimately improve patient care.
What are the classifications of medical devices according to the FDA?
The FDA categorizes medical devices into three classifications based on risk: Class I (low risk), Class II (moderate risk), and Class III (high risk).
What are the regulatory requirements for Class I, Class II, and Class III devices?
Class I products typically require basic regulations, Class II products often require a 510(k) premarket notification to demonstrate substantial equivalence to a legally marketed item, and Class III products require a more stringent Premarket Approval (PMA) process, which involves comprehensive clinical data to establish safety and efficacy.
Why is it important to understand FDA classifications for medical devices?
Understanding these classifications is crucial as they dictate the pathway for approval and the specific regulatory requirements that must be met for each class of device.
What is the 510(k) pathway and its significance?
The 510(k) pathway is a premarket notification process for Class II products that allows manufacturers to demonstrate that their device is substantially equivalent to a legally marketed device. It is significant because 81% of surgical instruments were approved via this pathway, although it has a higher recall rate compared to PMA.
How does the recall rate compare between 510(k) and PMA approved products?
The recall rate for products approved via the 510(k) pathway is 11.6%, while the recall rate for PMA approved products is significantly lower at 2.3%. Additionally, 510(k) product recalls are 5.32 times more likely than PMA product recalls.
What is the average time to receive a decision on FDA 510(k) applications?
The average time to receive a decision on FDA 510(k) applications is approximately 147 days.
Who can assist with navigating FDA device regulations?
Experts like Ana Criado, Director of Regulatory Affairs at bioaccess, have extensive experience in regulatory affairs and clinical trials, making it easier to navigate these complexities.
What clinical trial management services does bioaccess offer?
Bioaccess offers comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.