


The article underscores the critical significance of comprehending FDA medical device classification for clinical research directors. This understanding is essential as it profoundly influences regulatory pathways and market access. The classification system categorizes devices into Class I, II, and III based on risk levels, with each class imposing distinct regulatory requirements. These requirements directly impact approval processes, compliance strategies, and resource allocation for market entry, making it imperative for clinical research directors to be well-versed in this classification system.
Understanding the FDA classification system is essential for clinical research directors navigating the complex landscape of medical device approval. This system categorizes devices into three distinct classes based on risk and regulatory requirements, directly influencing product development strategies and market access.
With evolving regulations and the increasing demand for innovation, how can leaders ensure compliance while optimizing their pathways to market? This article delves into the intricacies of FDA classification, offering critical insights that empower clinical research directors to enhance their strategic approaches and successfully navigate the approval process.
The FDA classification of medical instruments is determined by their intended purpose and associated risk levels, which is a crucial process for establishing the necessary approvals for market entry. This classification system consists of three primary categories:
Understanding these categories is vital for clinical research leaders as it aligns product development strategies with compliance requirements. Recent trends indicate a growing emphasis on the importance of adhering to the FDA classification system, as it directly influences the approval process and market entry strategies for innovative medical products.
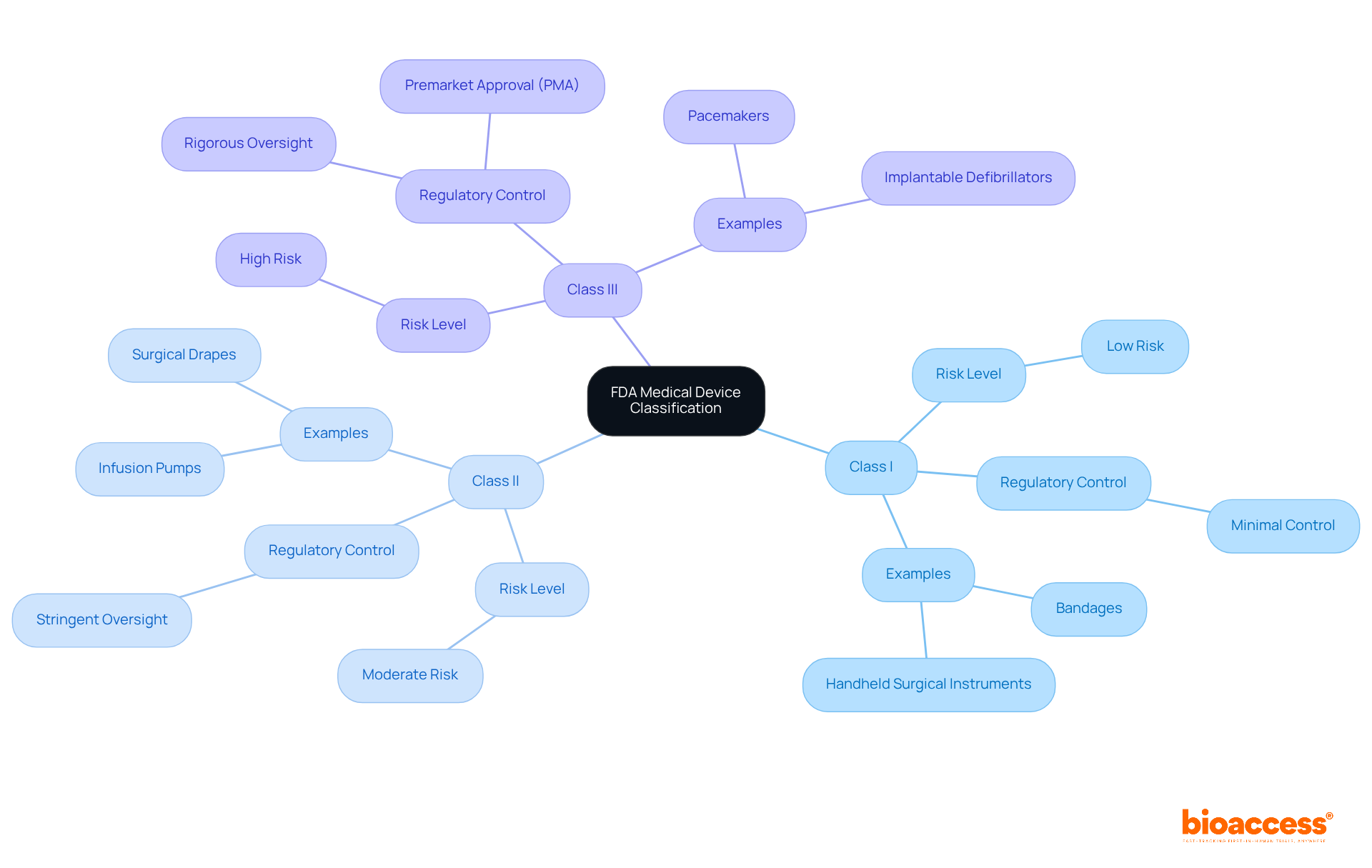
The FDA classifies medical devices into three distinct categories based on their risk levels and regulatory requirements:
Category I: These items are considered low risk and are typically exempt from premarket notification. They must adhere to general controls, which include proper labeling and compliance with manufacturing practices. Common examples include:
Category II: Indicating moderate risk, Category II instruments generally necessitate a 510(k) premarket notification to show substantial equivalence to a currently legally marketed instrument. This pathway is crucial for ensuring safety and effectiveness. Notable examples include:
Category III: These high-risk instruments require premarket approval (PMA) to confirm their safety and effectiveness. The PMA process is rigorous, often involving extensive testing and clinical trials. Examples of Class III devices include:
Understanding these classifications is vital for clinical research directors, as it informs the necessary regulatory steps for their devices. Insights from industry experts indicate that staying updated on FDA regulations and leveraging advancements in technology can significantly enhance the approval process for Class II devices, particularly as the landscape evolves in 2025. Bioaccess® offers accelerated clinical trial services that can help Medtech, Biopharma, and Radiopharma startups navigate these regulatory pathways efficiently.
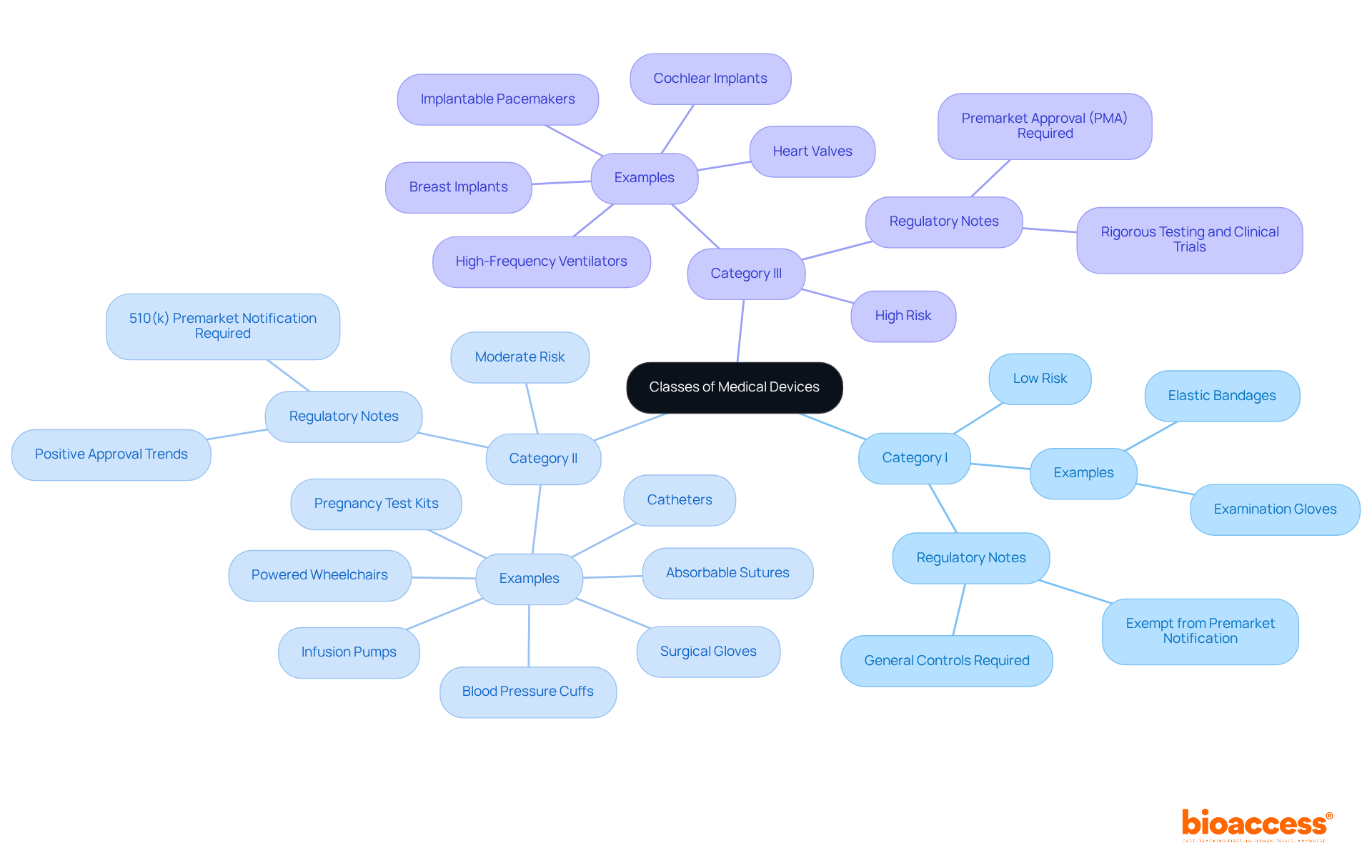
To effectively navigate the FDA classification process, it is essential to follow these steps:
Insights from compliance experts like Ana Criado, Director of Affairs at bioaccess, and Katherine Ruiz, a specialist in medical devices and in vitro diagnostics, provide valuable guidance. Ana's extensive experience in compliance matters and her consultancy role for global companies, along with Katherine's expertise in advising foreign manufacturers on market clearance in Colombia, underscore the importance of understanding both local and international frameworks. By adhering to these steps and utilizing expert knowledge, clinical research directors can efficiently navigate the FDA classification process, ensuring compliance and expediting the route to market.
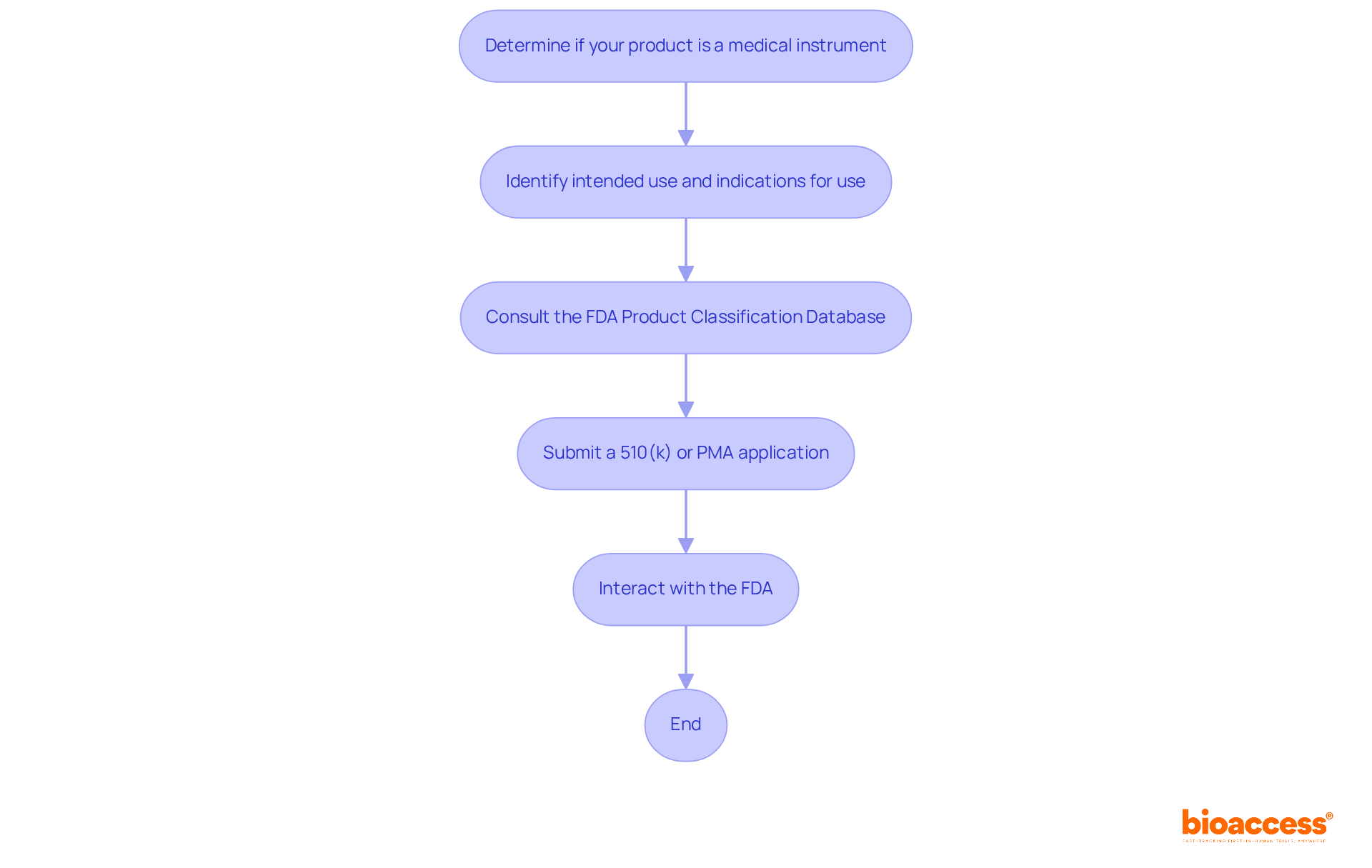
The FDA classification of a medical device significantly influences market access and compliance strategies.
The device's class dictates the FDA classification, which in turn affects the regulatory pathway for approval and impacts the time and resources needed for market entry. In Colombia, INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) supervises the regulation of medical products, ensuring adherence to health standards. The FDA classification indicates that Class I devices generally enjoy a quicker approval process, while Class III devices encounter more rigorous requirements, including extensive clinical data and post-market obligations. For example, the 510(k) pathway expenses vary from $100K to $2M, whereas the PMA pathway can vary from $5M to over $50M, emphasizing the financial consequences of categorization. INVIMA is designated as a Level 4 health authority by PAHO/WHO, highlighting its proficiency in oversight functions.
Market Approach: A comprehensive grasp of FDA classification is vital for developing a market approach that conforms to compliance expectations. For instance, Category II products frequently require clinical information to validate their assertions, which can influence marketing strategies and intended audiences. As Marco Theobold observes, 'Accurately recognizing the FDA classification of a product is not merely a regulatory requirement—it significantly impacts business strategy, R&D investment, market preparedness, and risk management.'
Post-Market Surveillance: Each class comes with distinct post-market surveillance requirements. Class III items, in particular, may require extensive monitoring and reporting to ensure ongoing safety and effectiveness, reflecting the increased risk associated with these products. Understanding the FDA classification of products is crucial, as it helps manufacturers identify product codes and applicable regulations based on a device's intended use. In Colombia, INVIMA's Directorate for Medical Devices and other Technologies plays a vital role in monitoring these aspects, ensuring that products meet the necessary safety and efficacy standards.
Recognizing these implications allows clinical research directors to strategically plan product development and market entry, ensuring compliance while optimizing the pathway to commercialization.
Understanding the FDA classification system for medical devices is essential for clinical research directors aiming to align product development with regulatory requirements. This classification not only categorizes devices based on their risk levels but also directly influences market access and compliance strategies. By grasping the distinctions between Class I, Class II, and Class III devices, professionals can strategically navigate the approval process and optimize their approach to market entry.
The article outlined the three primary classes of medical devices, emphasizing the regulatory implications tied to each category:
Furthermore, insights into the FDA classification process highlighted the importance of thorough preparation and proactive engagement with the FDA to facilitate timely approvals.
In light of the complexities involved in navigating the FDA classification system, it is crucial for clinical research directors to stay informed and utilize available resources effectively. As the landscape evolves, especially with anticipated changes in 2025, embracing a comprehensive understanding of these classifications will not only enhance compliance but also drive successful product commercialization. The significance of accurately recognizing and responding to FDA classifications cannot be overstated; it is a fundamental element that shapes business strategies and impacts the future of medical innovation.
What is the FDA classification system for medical devices?
The FDA classification system categorizes medical devices based on their intended purpose and associated risk levels, which is essential for determining the necessary approvals for market entry.
What are the three primary classes of medical devices according to the FDA?
The three primary classes are Class I (low-risk devices), Class II (moderate-risk devices), and Class III (high-risk devices).
What are examples of Class I medical devices?
Class I medical devices include low-risk items such as bandages and handheld surgical instruments, which have a minimal chance of causing contamination.
What distinguishes Class II medical devices from Class I?
Class II medical devices are moderate-risk items that require more stringent oversight to ensure safety and effectiveness, such as infusion pumps and surgical drapes.
What are some examples of Class III medical devices?
Class III medical devices are high-risk products that require the most rigorous oversight, including premarket approval. Examples include pacemakers and implantable defibrillators.
Why is understanding the FDA classification system important for clinical research leaders?
Understanding the FDA classification system is vital for clinical research leaders as it helps align product development strategies with compliance requirements, influencing the approval process and market entry strategies for innovative medical products.