


Successfully mastering FDA device approval requires a clear understanding of medical device classifications, navigating various approval pathways, and ensuring ongoing compliance post-approval. These steps are essential for anyone involved in clinical research, as they directly impact the success of medical devices in the market.
Thorough research and early engagement with the FDA are crucial; they not only enhance the chances of a successful application but also establish a foundation for maintaining product integrity.
Furthermore, robust quality management systems play a pivotal role in this process, reinforcing the importance of diligence and expertise in achieving compliance and fostering trust in the marketplace.
Navigating the intricate landscape of medical device regulation is a daunting endeavor, yet it is essential for ensuring safety and efficacy in healthcare products. This article serves as a comprehensive guide to mastering the FDA device approval process, outlining critical steps that can significantly enhance the likelihood of a successful application.
With multiple pathways available, such as 510(k), PMA, and De Novo, how can innovators effectively select the right route and prepare for the challenges that lie ahead?
Medical equipment regulation is governed by a comprehensive system designed to ensure security and effectiveness. Understanding this landscape is crucial for those involved in clinical research.
Definition of Medical Devices: A medical device encompasses a broad spectrum of instruments, apparatus, and software intended for medical purposes, including diagnostic, therapeutic, and monitoring functions. This definition sets the stage for understanding the regulatory environment.
Regulatory Bodies: The FDA device plays a pivotal role in overseeing the approval of medical equipment, ensuring that products meet stringent safety and effectiveness standards. Familiarity with the FDA's processes, as well as other relevant organizations, is essential for compliance and successful navigation of the regulatory landscape.
Classification of Equipment: Medical instruments are categorized into three groups based on risk levels:
Class I products, such as adhesive bandages and manual stethoscopes, typically feature simple designs and pose minimal risk. In contrast, Class II products, which represent 43% of all medical equipment, include more complex items like endoscopes and powered wheelchairs. Class III products, such as cardiac pacemakers, are high-risk and often require extensive regulatory scrutiny. This classification underscores the importance of understanding the specific requirements for each category.
Importance of Compliance: Adhering to regulatory requirements is vital to avoid legal repercussions and ensure patient safety. A thorough grasp of the nuances of FDA device regulations, along with recent updates and guidance on human factors documentation, is essential for successful product authorization.
By mastering these fundamentals, you will be better prepared to navigate the complexities of the FDA device authorization process and appreciate the rationale behind various regulatory requirements.

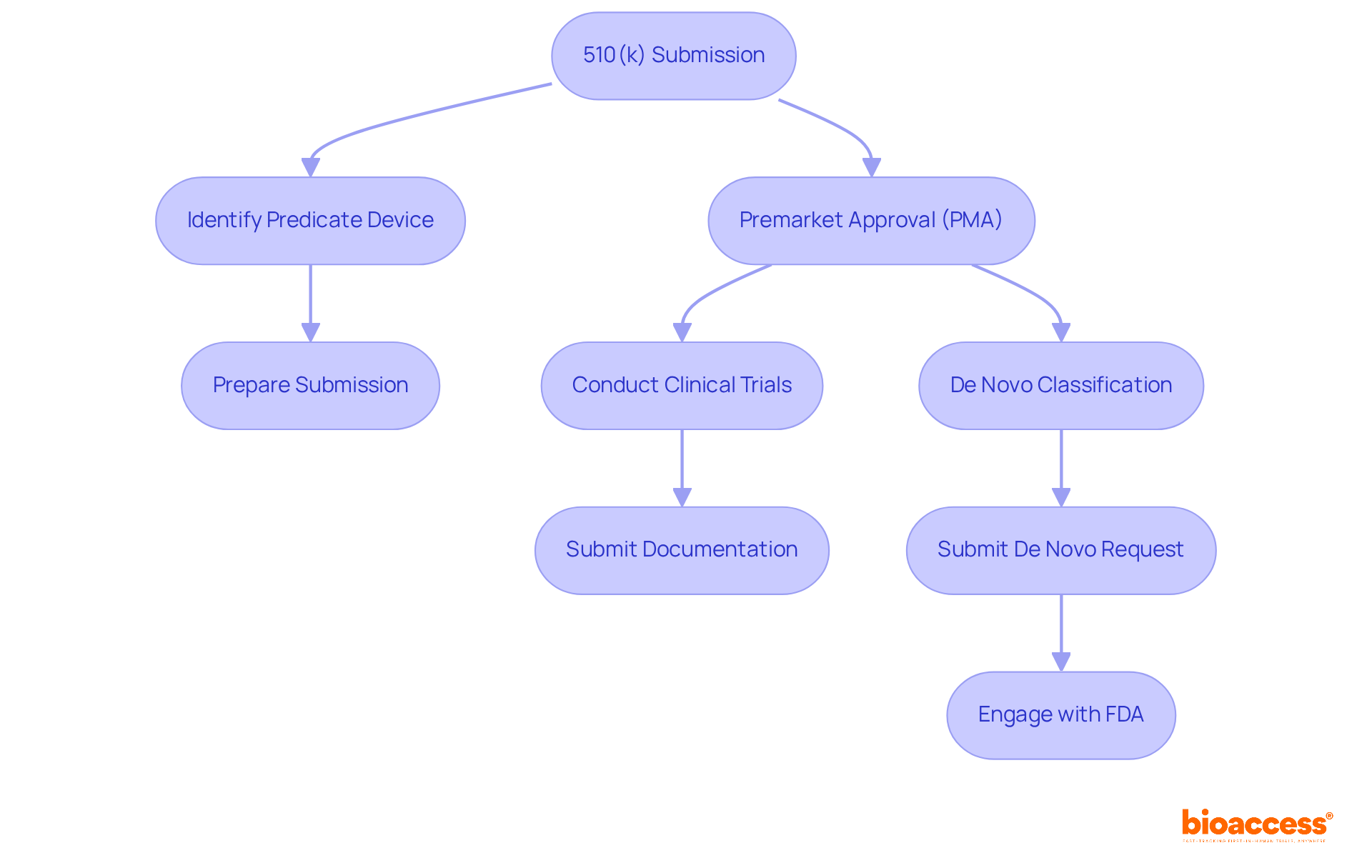
The FDA offers several pathways for medical device approval, which are crucial for navigating the regulatory landscape effectively:
510(k) Submission: This pathway is designed for devices that are substantially equivalent to an already marketed device. Key steps include identifying a predicate device and preparing a comprehensive submission that demonstrates equivalence.
Premarket Approval (PMA): Required for high-risk products, this pathway involves a rigorous review process. Steps include conducting clinical trials to provide evidence of safety and effectiveness, followed by submitting detailed documentation for FDA review.
De Novo Classification: This pathway applies to new products that are low to moderate risk but lack a predicate. Steps include submitting a De Novo request with supporting data and engaging in discussions with the FDA to clarify requirements.
Grasping these pathways enables creators to select the most effective path for their product, ensuring adherence and expediting the assessment process.
To enhance your chances of a successful FDA application, consider the following strategies:
These strategies will help you prepare a strong application that meets FDA standards and facilitates a smoother approval process while positioning your product effectively in the Latin American market.

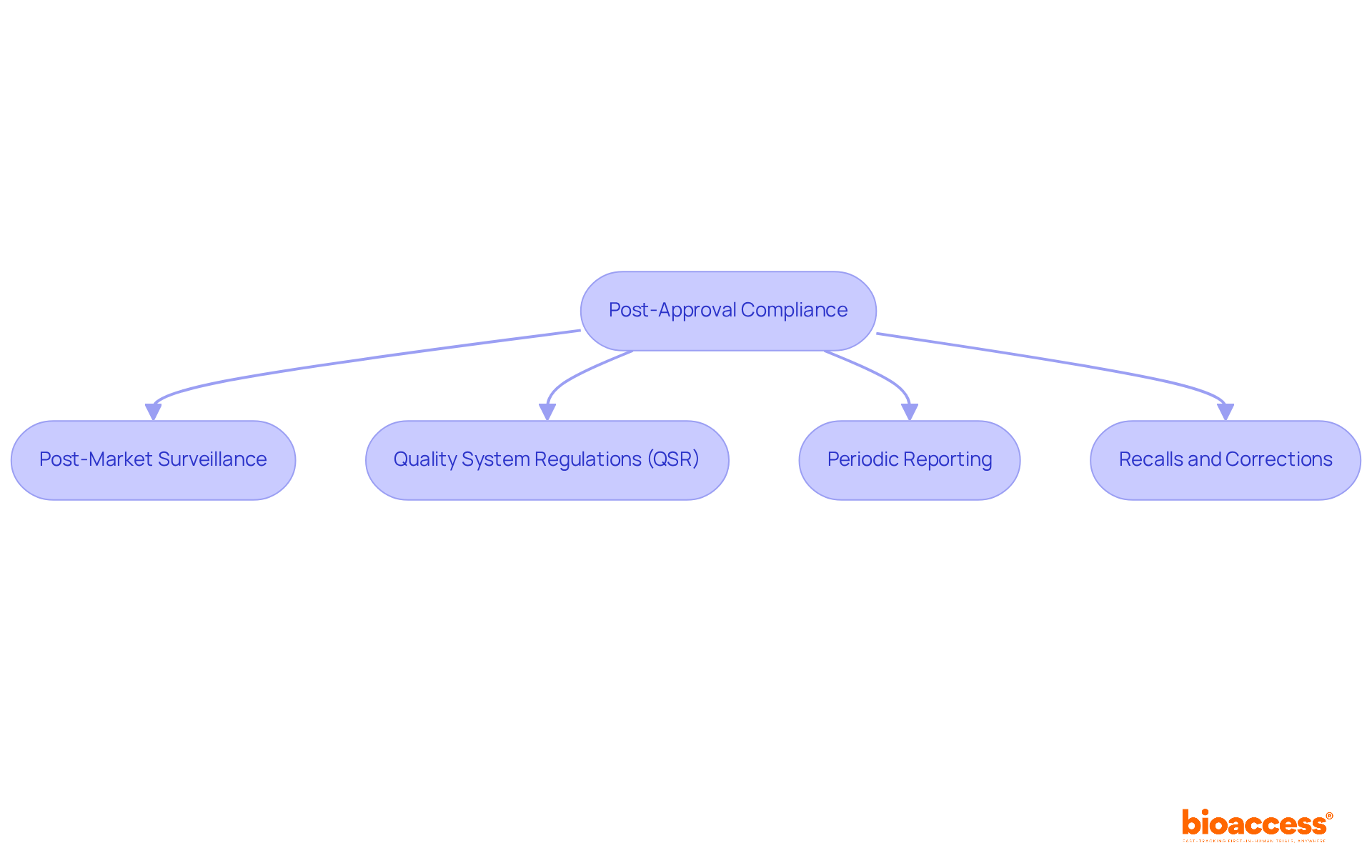
Once your equipment is approved, continuous adherence is crucial for upholding market integrity and guaranteeing patient well-being. Key aspects include:
Post-Market Surveillance: Establish robust systems to monitor the device's performance in the market. This encompasses gathering and examining adverse event reports, which typically amount to approximately 1,000 occurrences each year for medical apparatus, along with user input to recognize possible risk issues.
Quality System Regulations (QSR): Adhere to QSR to ensure that manufacturing processes consistently meet the standards required for an FDA device. Adhering to these regulations is not just a requirement but a dedication to quality and security that can improve product reliability and market trust.
Periodic Reporting: Be ready to submit regular reports regarding the FDA device, outlining any changes in performance or concerns regarding security. This involves updating post-market surveillance reports (PMSR) at least every three years for Class I items and annually for Class III items, ensuring that any emerging risks are swiftly addressed.
Recalls and Corrections: Understand the procedures for addressing issues that arise post-approval, including effective management of recalls. A proactive approach to risk management is essential, as it enables manufacturers to react quickly to any negative events, thereby safeguarding patient well-being and ensuring compliance.
By prioritizing post-approval compliance, companies can safeguard their devices' market presence and contribute to ongoing patient safety. This reinforces the notion that regulatory compliance is an ongoing commitment to product quality and innovation.
Mastering the FDA device approval process is crucial for anyone involved in the development of medical equipment. A comprehensive understanding of regulatory requirements—from the definition and classification of medical devices to the various approval pathways—establishes the foundation for a successful navigation of the FDA's rigorous evaluation process. This knowledge not only facilitates compliance but also enhances the safety and effectiveness of medical devices available in the market.
Key insights highlighted throughout the article include:
By engaging early with the FDA, compiling comprehensive documentation, and maintaining rigorous quality management systems, manufacturers can significantly enhance their likelihood of successful device approval. Furthermore, ongoing post-market surveillance and adherence to quality system regulations are essential for ensuring continuous compliance and safeguarding patient safety.
Ultimately, the journey of medical device approval is complex yet rewarding. By prioritizing regulatory compliance and grasping the nuances of the approval process, stakeholders can drive innovation in healthcare and improve patient outcomes. Embracing these strategies and insights will not only streamline the approval process but also reinforce a steadfast commitment to quality and safety within the medical device industry.
What is the definition of a medical device?
A medical device encompasses a broad spectrum of instruments, apparatus, and software intended for medical purposes, including diagnostic, therapeutic, and monitoring functions.
What role does the FDA play in medical device regulation?
The FDA plays a pivotal role in overseeing the approval of medical equipment, ensuring that products meet stringent safety and effectiveness standards.
How are medical devices classified based on risk levels?
Medical devices are categorized into three groups based on risk levels: Class I (low-risk), Class II (medium-risk), and Class III (high-risk).
Can you provide examples of each class of medical devices?
Class I products include items like adhesive bandages and manual stethoscopes. Class II products, which represent 43% of all medical equipment, include more complex items like endoscopes and powered wheelchairs. Class III products, such as cardiac pacemakers, are high-risk devices that require extensive regulatory scrutiny.
Why is compliance with medical device regulations important?
Adhering to regulatory requirements is vital to avoid legal repercussions and ensure patient safety. A thorough understanding of FDA device regulations and recent updates is essential for successful product authorization.
How can understanding medical device regulations benefit those involved in clinical research?
Mastering the fundamentals of medical device regulation prepares individuals to navigate the complexities of the FDA device authorization process and appreciate the rationale behind various regulatory requirements.