


The primary objective of this article is to elucidate the effective mastery of the FDA Fast Track Designation, a crucial element for enhancing success in clinical research. This overview delineates the purpose and advantages of this designation, outlines the eligibility criteria, describes the application process, and presents strategies for successful implementation. Emphasis is placed on the necessity of robust data and proactive communication with the FDA, which are essential to expedite the development of critical therapies for serious conditions.
The FDA Fast Track Designation serves as a pivotal mechanism within the pharmaceutical landscape, specifically engineered to expedite the development of therapies targeting serious health conditions and unmet medical needs. This designation not only streamlines the regulatory process but also fosters enhanced communication between drug developers and the FDA, thereby facilitating quicker access to life-saving treatments.
Nonetheless, navigating the complexities of this expedited pathway presents significant challenges for companies striving to comply with stringent requirements and timelines.
How can organizations effectively leverage the Fast Track Designation to maximize their chances of clinical research success while overcoming these hurdles?
The FDA Fast Track Designation serves as a pivotal procedure aimed at promoting the development and expediting the evaluation of medications designed to address severe conditions and fulfill unmet medical needs. The FDA fast track designation facilitates more frequent communication with the FDA, allowing sponsors to engage in discussions regarding their development plans and receive essential guidance on the regulatory process. Ultimately, the goal is to deliver critical new therapies to patients promptly, thereby enhancing patient outcomes and tackling significant health challenges.
The Accelerated Pathway proves particularly advantageous for medications demonstrating the potential to meet substantial health requirements, significantly reducing the time to market.
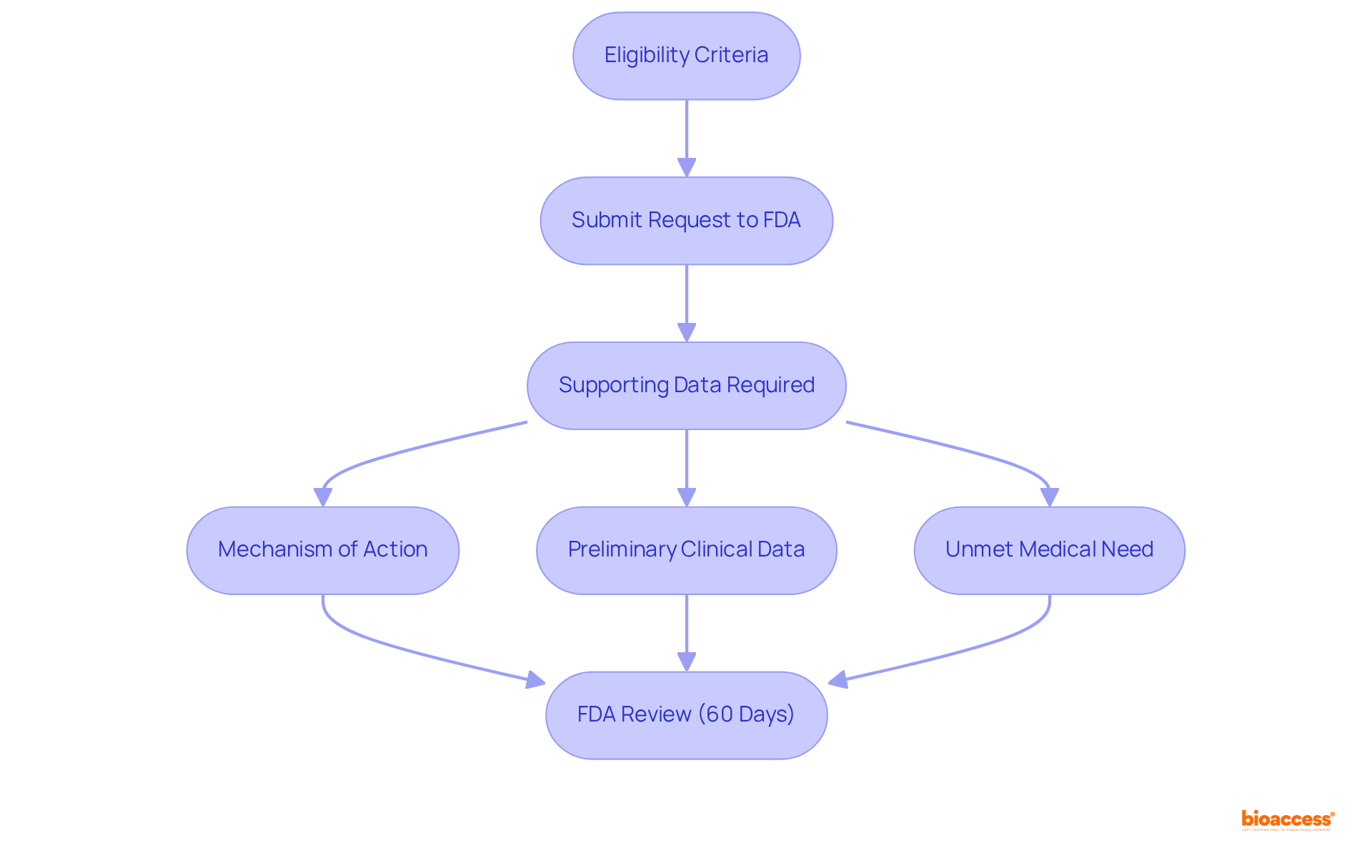
To qualify for Fast Track Designation, a medication must be intended to treat a serious condition and demonstrate the potential to address an unmet medical need. The application process typically commences with the submission of a request to the FDA, which can occur at any stage during the development process, including the Investigational New Product (IND) application phase. This request should encompass supporting data that details the substance's mechanism of action, preliminary clinical data, and the unmet medical need it seeks to fulfill. The FDA is committed to reviewing these requests within 60 days, offering feedback and guidance to the sponsor.
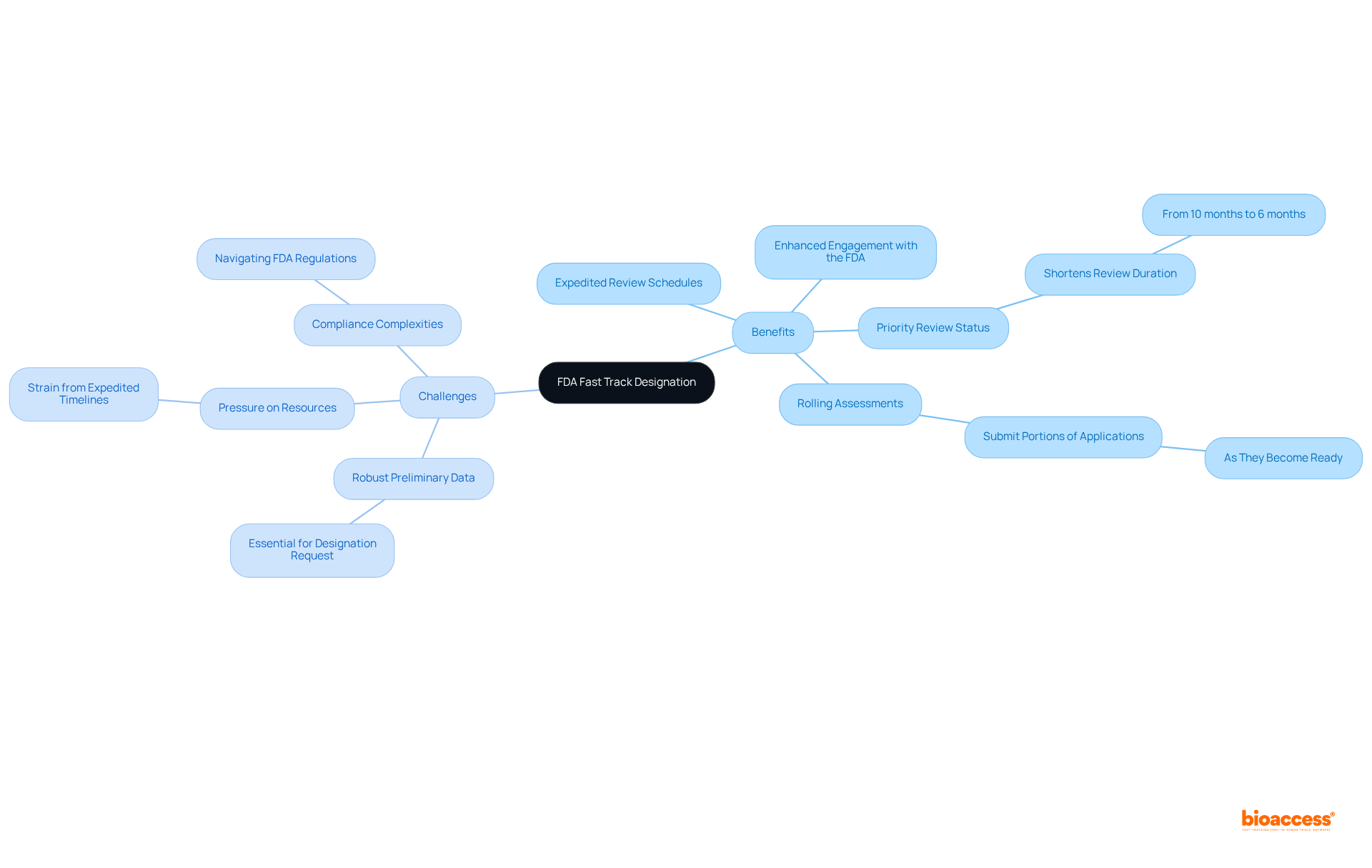
The advantages of FDA fast track designation are significant, such as:
However, challenges persist:
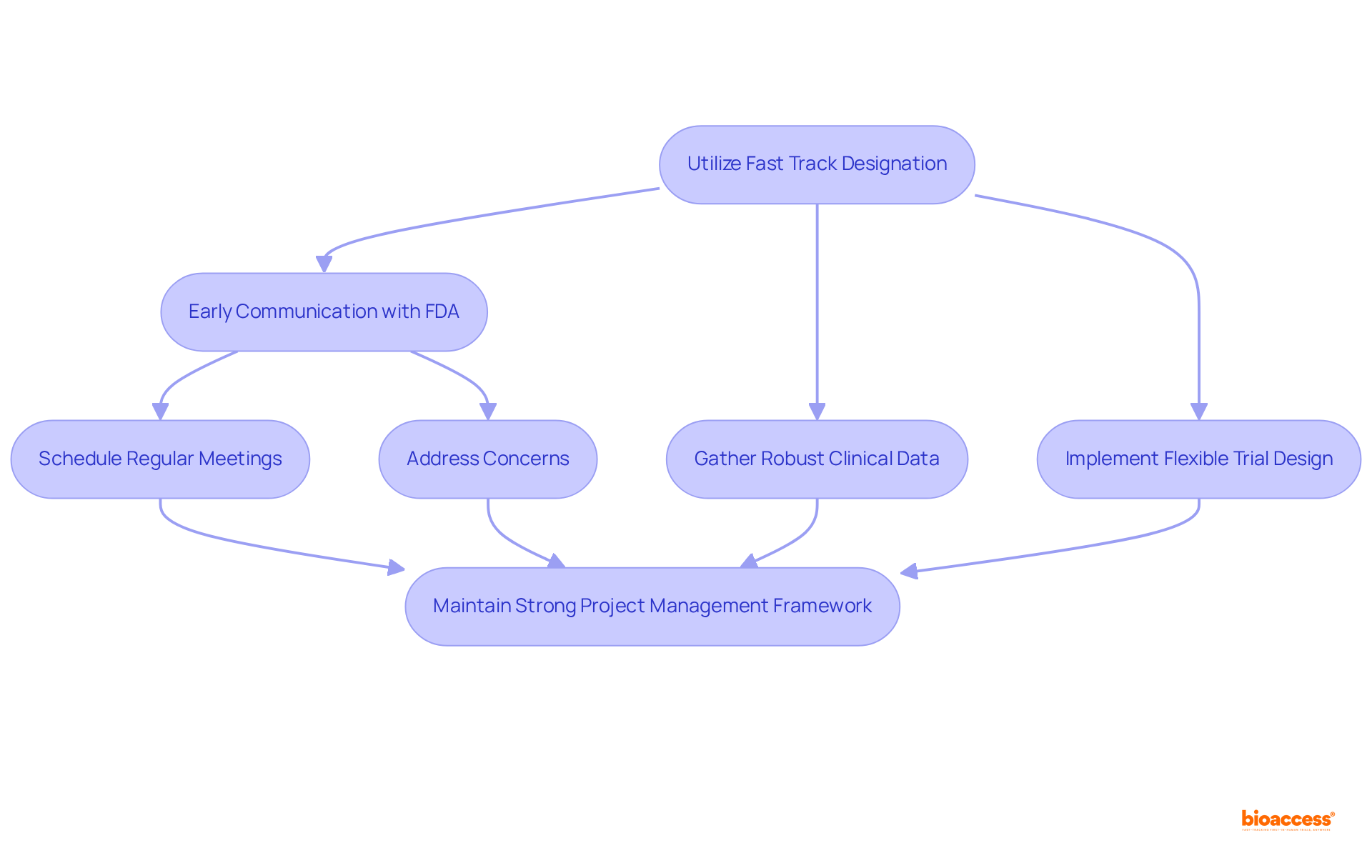
To effectively utilize Fast Track Designation, companies must prioritize early and continuous communication with the FDA. This proactive approach involves:
Furthermore, companies should concentrate on gathering robust clinical data that clearly demonstrates the drug's potential benefits and addresses the unmet medical need. Implementing a flexible trial design can also prove advantageous, allowing for necessary adjustments based on interim results. Ultimately, maintaining a strong project management framework is crucial to ensure that timelines are met and resources are allocated efficiently, thereby facilitating a smoother path to market.
The FDA Fast Track Designation serves as a pivotal mechanism for accelerating the development and review of essential therapies aimed at serious health conditions. By streamlining communication between sponsors and the FDA, this designation not only facilitates quicker access to vital treatments but also underscores the significance of addressing unmet medical needs within the healthcare landscape.
This article explored key aspects of the Fast Track Designation, including:
However, it is crucial to acknowledge the challenges associated with this designation, including:
Effective strategies for utilization, such as proactive communication and adaptable trial designs, were highlighted as essential for successfully navigating this intricate process.
In conclusion, the importance of the FDA Fast Track Designation cannot be overstated. It acts as a lifeline for patients in need of timely therapies and emphasizes the critical role of innovation in clinical research. By leveraging this designation effectively, stakeholders can contribute to a healthcare system that prioritizes patient outcomes and addresses pressing medical challenges. Embracing these strategies may ultimately lead to a more efficient pathway for bringing groundbreaking treatments to market, thereby enhancing the overall well-being of society.
What is FDA Fast Track Designation?
FDA Fast Track Designation is a procedure designed to promote the development and expedite the evaluation of medications that address severe conditions and fulfill unmet medical needs.
What is the purpose of FDA Fast Track Designation?
The purpose of FDA Fast Track Designation is to facilitate more frequent communication with the FDA, allowing sponsors to discuss their development plans and receive guidance on the regulatory process, ultimately aiming to deliver new therapies to patients more quickly.
How does FDA Fast Track Designation benefit medication development?
It benefits medication development by reducing the time to market for drugs that demonstrate the potential to meet significant health requirements, thereby enhancing patient outcomes and addressing major health challenges.