


This article delves into the critical mastery of FDA labeling guidance for medical devices, emphasizing the necessity of understanding regulations, required label elements, and compliance strategies. A thorough knowledge of FDA requirements is paramount, including the inclusion of device names, intended use, and unique identifiers. To navigate compliance challenges effectively, strategies such as:
are essential. These approaches not only help avoid common pitfalls but also ensure safety and adherence to regulations, reinforcing the importance of meticulous compliance in the Medtech landscape.
Navigating the complex landscape of FDA labeling requirements for medical devices is crucial for manufacturers seeking to ensure compliance and safeguard patient safety. This article explores the essential elements of FDA labeling guidance, providing a clear roadmap for understanding regulations and implementing effective labeling strategies.
With evolving standards and common pitfalls at every turn, how can manufacturers ensure their labels not only meet the stringent demands of the FDA but also enhance usability for healthcare providers and patients alike?
To effectively master the FDA labeling guidance, begin by familiarizing yourself with the FDA's regulations concerning medical products. The FDA delineates specific requirements in 21 CFR Part 801, encompassing general labeling standards alongside particular provisions for various categories of equipment. Key aspects to focus on include:
By thoroughly grasping these criteria, you will be better positioned to produce compliant labels that align with FDA labeling guidance.
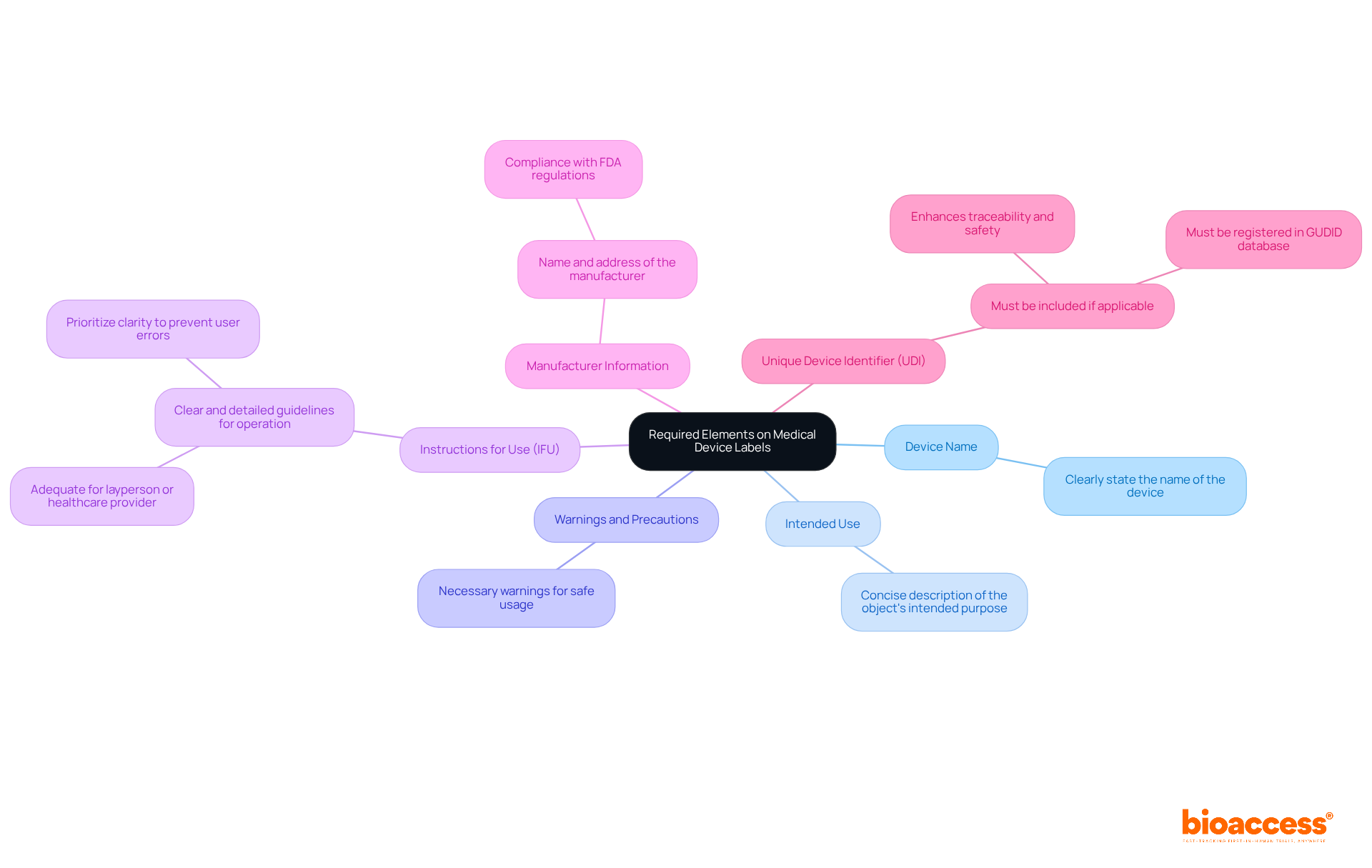
Grasping the FDA labeling guidance is essential for guaranteeing adherence and safety in medical equipment manufacturing. As a regulatory affairs expert, Katherine Ruiz emphasizes the importance of including the following elements on your medical device labels:
Creating a checklist of these elements can facilitate the development of compliant labels according to the FDA labeling guidance, ensuring that all necessary information is prominently displayed and easily readable. This approach not only meets regulatory standards but also enhances patient safety and device effectiveness. Furthermore, it is crucial to guarantee that markings are clear, noticeable, and suitably positioned, as outlined in Section 801.15 of the FDA regulations. Remember that updates post-clearance must be tracked and submitted via FDA’s established change protocols.
To ensure compliance with FDA labeling requirements, consider implementing the following strategies:
Develop a Labeling Plan: Create a comprehensive labeling plan that outlines all required elements, such as product identity, net contents, ingredient declarations, and warnings, along with timelines for label development. This structured approach helps in maintaining clarity and consistency.
Conduct Regular Reviews: Schedule regular assessments of packaging materials to ensure they remain compliant with the FDA labeling guidance and any updates to FDA regulations. Identification mistakes are among the top five most frequent Form 483 citations issued to medical device companies, and over 50% of medication use errors are associated with inadequate identification. Proactive evaluations can significantly reduce risks.
Engage Cross-Functional Teams: Collaborate with regulatory, quality assurance, and marketing teams to ensure all perspectives are taken into account in the packaging process. This partnership promotes a thorough comprehension of compliance requirements and improves the quality of the markings.
Utilize Tagging Software: Consider using specialized tagging software that can streamline the creation and management of compliant tags. Statistics show that automated quality control tools can decrease review times by as much as 89%, greatly improving efficiency and precision in the tagging process.
Training and Education: Provide training for your team on FDA labeling guidance and best practices to foster a culture of compliance. Regular training sessions can help keep your team aware of the latest regulations and enhance overall accuracy in markings.
By applying these strategies, you can improve your tagging process and decrease the chances of compliance problems, ultimately guaranteeing that your medical devices meet FDA standards and are safe for consumer use.

Even with a solid understanding of FDA labeling guidance and a well-structured labeling plan, pitfalls can still occur. Awareness of these common compliance issues is crucial for maintaining adherence to regulations:
By being vigilant about these pitfalls and actively working to address them, you can significantly enhance your compliance with the FDA labeling guidance.

Mastering FDA labeling guidance for medical devices is an essential endeavor for manufacturers aiming to ensure compliance and enhance patient safety. Understanding the regulatory framework and implementing effective labeling strategies enables companies to produce labels that not only meet legal requirements but also provide critical information to users.
This article emphasizes key components such as:
It highlights the importance of clarity in device naming, intended use, and necessary warnings. Furthermore, it advocates for the development of structured labeling plans, regular reviews, and cross-functional collaboration to streamline the labeling process.
In light of these insights, it is crucial for medical device manufacturers to prioritize adherence to FDA labeling guidance. By doing so, they mitigate compliance risks and foster a culture of safety and reliability in their products. Embracing these practices ultimately contributes to better health outcomes and builds trust with consumers, reinforcing the significance of diligent labeling in the medical device industry.
What are the FDA labeling requirements for medical devices?
The FDA labeling requirements for medical devices are outlined in 21 CFR Part 801, which includes general labeling standards and specific provisions for different categories of equipment.
What qualifies as a medical device under FDA regulations?
A medical device is defined by the FDA as any instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or similar article that is intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Why is it important to specify the intended use of medical equipment?
Specifying the intended use of medical equipment is crucial because it influences the labeling criteria and ensures that the device is used appropriately in accordance with its purpose.
What should I review to ensure compliance with FDA labeling guidance?
You should review the specific sections of the Code of Federal Regulations (CFR) that pertain to your type of equipment and familiarize yourself with relevant industry standards that may apply to your product's markings.
How can I ensure my labels are compliant with FDA regulations?
To ensure your labels are compliant, you should thoroughly understand the FDA's labeling guidance, including the definition of a medical device, labeling regulations, intended use, and adherence to applicable industry standards.