


The article underscores the critical importance of mastering FDA labeling requirements for medical devices, a necessity for ensuring compliance and enhancing patient safety. It highlights that non-compliance can result in severe repercussions, including:
This emphasizes the imperative for manufacturers to strictly adhere to these regulations, protecting not only their products but also public health.
Medical device labeling is crucial for ensuring user safety and compliance in the expansive $577 billion medical equipment industry. As manufacturers navigate the complexities of FDA regulations, the stakes are high; improper labeling can lead to severe consequences, including regulatory actions, legal liabilities, and reputational damage. This raises a pressing question: how can manufacturers effectively master these intricate labeling requirements to safeguard their products and maintain a strong market presence?
Medical equipment markings encompass essential information accompanying medical items, including usage instructions, safety cautions, and other vital details. Their primary functions are to ensure user safety, provide necessary guidance for proper device usage, and meet regulatory requirements. Effective identification is crucial in preventing misuse, thereby significantly enhancing patient safety and ensuring compliance with legal standards.
In clinical research, precise identification is vital for maintaining study integrity and safeguarding participant health. Notably, marking mistakes rank among the top five most frequent citations issued to medical equipment companies by the FDA, underscoring the critical importance of meticulous marking practices.
Furthermore, the global medical equipment sector, valued at approximately $577 billion, necessitates strict identification protocols to facilitate market entry and adherence. Understanding the complexities of medical product identification is essential for complying with the FDA labeling requirements for medical devices and achieving successful market access.
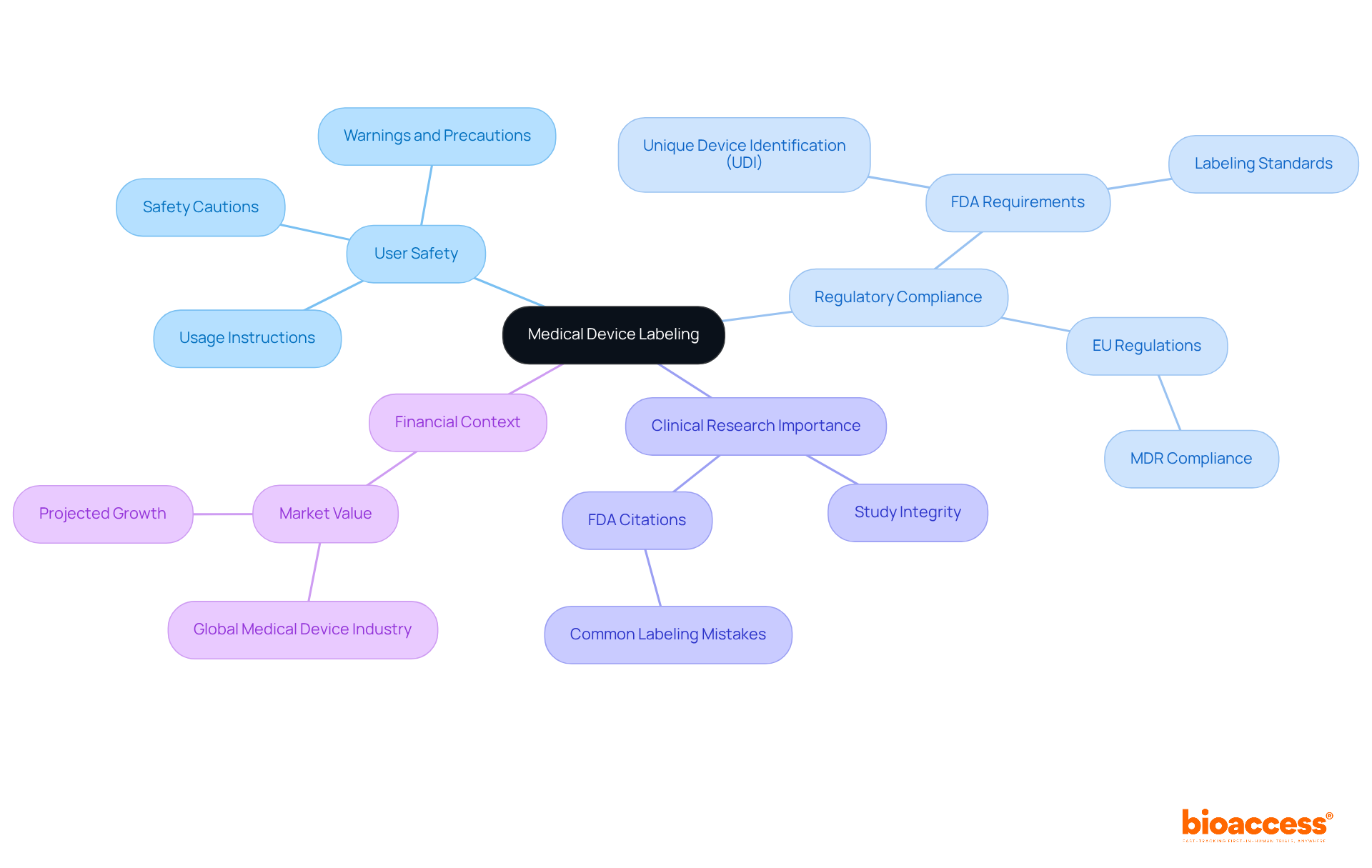
The FDA has established specific regulations governing medical apparatus markings, primarily found in Title 21 of the Code of Federal Regulations (CFR). Key regulations include:
21 CFR Part 801: This part outlines the general labeling requirements for medical devices, emphasizing the necessity for adequate directions for use and warnings. Adhering to these standards is essential, as marking mistakes rank among the top five most frequent causes for FDA warning letters.
21 CFR Part 820: This section addresses the Quality System Regulation (QSR), requiring that marking be incorporated into the apparatus's design controls. This integration is crucial for upholding regulations and ensuring that markings stay precise throughout the product lifecycle.
21 CFR Part 812: This section relates to investigational equipment, necessitating particular markings for clinical trials to guarantee that all essential information is communicated to participants and researchers.
Understanding the FDA labeling requirements for medical devices is crucial for manufacturers to ensure compliance and avoid potential penalties or product recalls. Frequent updates and guidance materials from the FDA should be observed to remain aware of any modifications in requirement standards, as the environment is constantly changing to improve product safety and efficacy. Furthermore, manufacturers must be aware of unique product identification (UDI) requirements, which are essential for contemporary medical product regulation. With experts like Ana Criado and Katherine Ruiz, who have extensive experience in regulatory affairs and biomedical engineering, manufacturers can navigate these complex requirements more effectively.
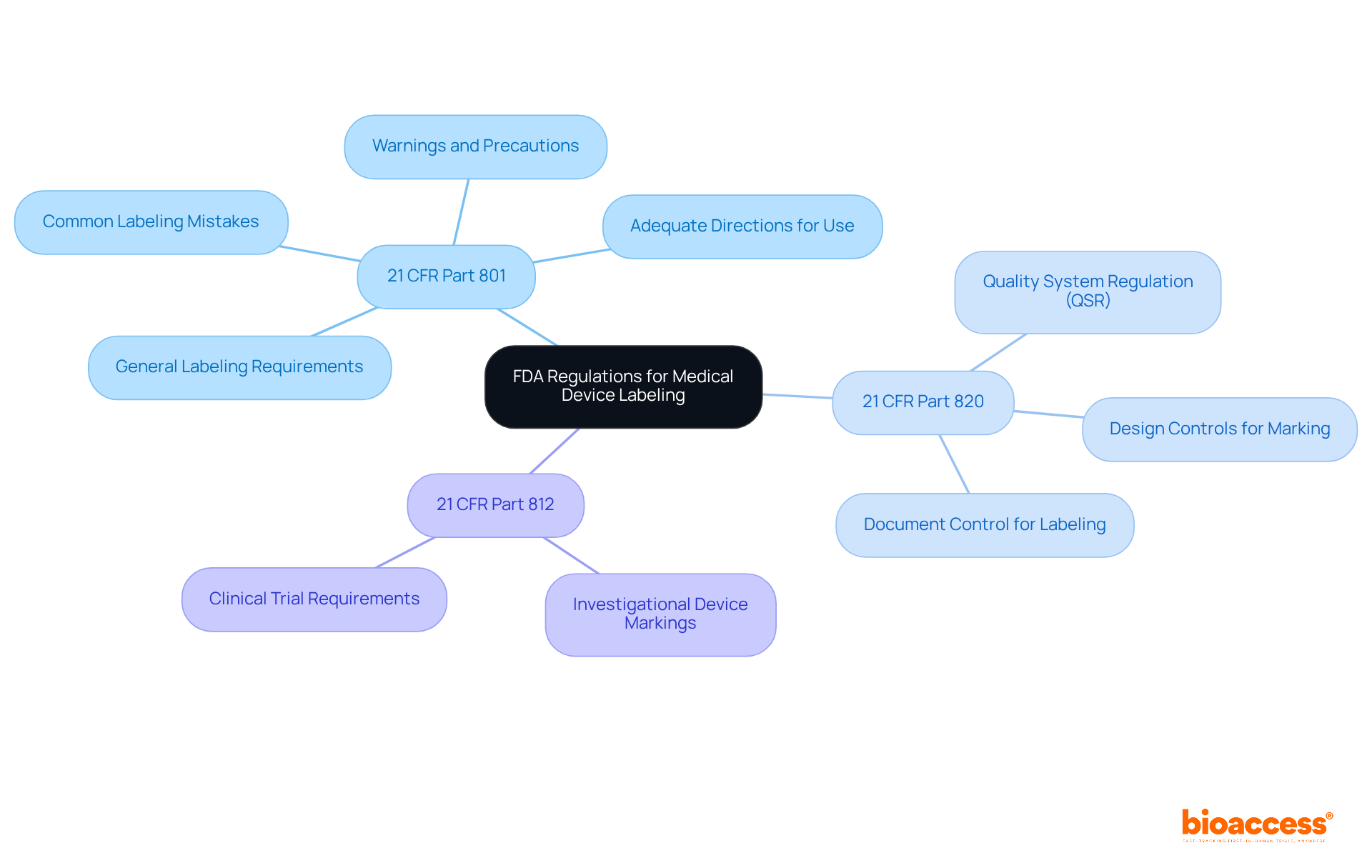
To achieve compliance with FDA labeling requirements for medical devices, manufacturers must prioritize several key elements and best practices.
Clear and Concise Language: Utilize straightforward language that is easily comprehensible to the intended audience, avoiding unnecessary technical jargon to enhance accessibility.
Comprehensive Instructions: Provide detailed guidelines for use, encompassing preparation, operation, and maintenance of the equipment, ensuring users can handle it safely and effectively.
Safety Information: Clearly communicate any potential risks or side effects associated with the apparatus, including appropriate warnings to safeguard users. It is crucial to emphasize that misuse of medical devices can lead to serious injury or even death, underscoring the importance of effective labeling.
Label Format and Design: Ensure labels are legible by utilizing appropriate font sizes and contrast. Labeling must be prominent and appropriately placed as stated in Section 801.15. Incorporate symbols and graphics where appropriate to enhance understanding and adherence.
Manufacturer Information: FDA-compliant labels must include the manufacturer's name and business location, which is essential for compliance and fostering user trust.
Review and Testing: Conduct usability testing to verify that users can easily interpret and follow instruction guidelines. Labeling errors are a common cause of FDA warning letters, making this step critical. Regularly review and update labels based on user feedback and evolving regulatory requirements.
Applying these best practices not only improves the effectiveness of labeling but also ensures adherence to FDA labeling requirements for medical devices, ultimately reducing the risk of misuse and enhancing patient safety. Effective labeling methods assist in reducing negative experiences for users, emphasizing the necessity for thorough adherence.
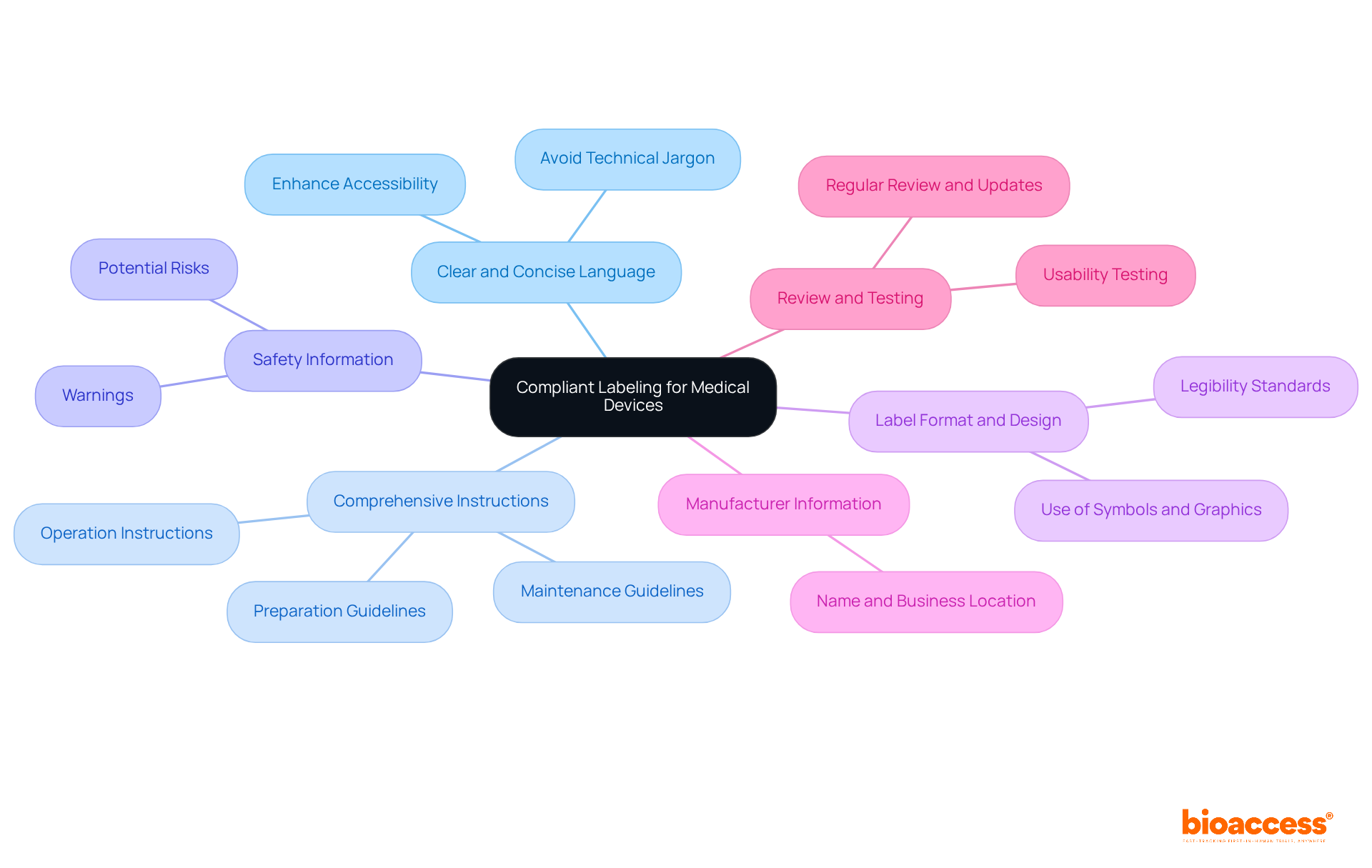
Non-compliance with FDA labeling requirements can lead to significant repercussions, including:
Regulatory Actions: The FDA possesses the authority to issue warning letters, impose fines, or initiate product recalls when labeling discrepancies are identified. In fiscal year 2021, the number of warning letters sent to manufacturers reached an alarming high, underscoring the agency's intensified scrutiny of regulatory concerns. Notably, 49.1% of eligible firms failed to submit field alert reports to the FDA from FY2018 to FY2021, highlighting the critical importance of compliance and the reporting of significant quality issues.
Legal Responsibility: Manufacturers may face lawsuits from patients or healthcare providers if inaccurate descriptions result in harm or adverse outcomes. Statistics reveal that labeling errors are a prevalent cause for FDA warning letters, accentuating the legal risks associated with non-compliance. In FY2021, the percentage of non-compliant samples surged to 35%, a significant increase from 16% in FY2020, reflecting the mounting challenges manufacturers face in adhering to standards.
Market Access Issues: Non-compliance can lead to delays or denials of product approvals, severely impeding market entry and disrupting business operations. Incomplete or ambiguous markings have been shown to extend the review process, adversely affecting a company's ability to launch products promptly. Additionally, the FDA's New Inspection Protocol Project aims to enhance data collection and understanding of quality factors influencing drug production, which could impact regulatory outcomes.
Reputation Damage: Companies risk incurring reputational harm, which can undermine consumer trust and negatively affect future sales. The rise in recall events, particularly Class I recalls, which escalated from 300 in 2019 to 800 in FY2021, serves as a cautionary tale for manufacturers regarding the long-term consequences of non-compliance. Furthermore, adhering to ISO standards, such as ISO 13485:2016 for marking control and ISO 15223-1 for acceptable symbols and presentation standards, is essential for upholding regulations and ensuring product integrity.
Understanding these consequences underscores the critical need for strict adherence to FDA labeling requirements, not only to ensure regulatory compliance but also to protect the success and integrity of medical device products.
Mastering FDA labeling requirements for medical devices is crucial for ensuring compliance, enhancing patient safety, and facilitating market entry. The significance of precise labeling cannot be overstated; it plays a vital role in guiding users, preventing misuse, and adhering to regulatory standards. Manufacturers must recognize that effective labeling is not merely a legal obligation but a fundamental aspect of product integrity and patient well-being.
Key insights throughout this discussion highlight the importance of understanding FDA regulations, particularly those outlined in Title 21 of the CFR, and implementing best practices for compliant labeling. By emphasizing clear communication, comprehensive instructions, and rigorous testing, manufacturers can significantly mitigate the risk of labeling errors that may lead to regulatory actions, legal liabilities, and reputational damage. The consequences of non-compliance, including increased scrutiny from the FDA and potential market access challenges, further underscore the necessity for diligence in labeling practices.
Given these considerations, it is imperative for medical device manufacturers to prioritize adherence to FDA labeling requirements. By staying informed about current regulations and continuously refining labeling strategies, companies can not only safeguard their products and patients but also cultivate trust and credibility within the healthcare community. Taking proactive steps towards compliance ensures that medical devices fulfill their intended purpose safely and effectively, ultimately contributing to improved health outcomes for all.
What is medical device labeling?
Medical device labeling refers to the essential information that accompanies medical items, including usage instructions, safety cautions, and other vital details necessary for the safe and effective use of the device.
Why is medical device labeling important?
Medical device labeling is important because it ensures user safety, provides necessary guidance for proper device usage, and meets regulatory requirements. It helps prevent misuse and enhances patient safety while ensuring compliance with legal standards.
How does labeling impact clinical research?
In clinical research, precise labeling is vital for maintaining study integrity and safeguarding participant health. Accurate identification helps ensure that devices are used correctly and that study results are reliable.
What are the consequences of marking mistakes in medical devices?
Marking mistakes are among the top five most frequent citations issued to medical equipment companies by the FDA, highlighting the critical importance of meticulous marking practices to avoid regulatory issues.
What is the value of the global medical equipment sector?
The global medical equipment sector is valued at approximately $577 billion, which necessitates strict identification protocols for market entry and compliance with regulations.
What do manufacturers need to comply with regarding medical device labeling?
Manufacturers need to understand the complexities of medical product identification to comply with FDA labeling requirements for medical devices and achieve successful market access.