


The article delineates a structured approach to mastering feasibility clinical trial assessments in four pivotal steps. It emphasizes the evaluation of:
By highlighting the critical role of these assessments in identifying potential obstacles and refining recruitment strategies, the article demonstrates how comprehensive feasibility evaluations can significantly mitigate risks and enhance the likelihood of successful clinical studies.
The success of clinical trials hinges on a crucial yet often overlooked component: feasibility assessments. These evaluations not only determine the practicality of conducting a study but also help identify potential hurdles that could derail research efforts. As the stakes rise—given that nearly 80% of medical studies fail to meet enrollment targets—understanding the intricacies of feasibility assessments becomes paramount. What strategies can researchers employ to navigate these challenges and ensure their trials are set up for success?
The evaluation of both the practicality and likelihood of successfully conducting a clinical study is known as a feasibility clinical trial. This multifaceted process includes assessing:
A thorough feasibility clinical trial evaluation is essential for identifying potential obstacles, ensuring that studies can be conducted ethically and efficiently. It represents a pivotal step in the planning phase, empowering researchers to make informed decisions regarding whether to proceed with the study or modify its design.
Given that approximately 80% of medical studies fail to meet initial enrollment targets, a comprehensive feasibility clinical trial assessment significantly mitigates risks associated with recruitment delays, which can cost drug development firms up to $8 million daily. Furthermore, successful feasibility clinical trials are particularly critical in the Medtech and Biopharma sectors, where the complexity of studies has escalated, with eligibility criteria increasing by 61% from 2001 to 2015.
bioaccess® offers extensive management services for feasibility clinical trials, including:
These services are specifically tailored for the Latin American market. The methodologies employed in these feasibility studies encompass comprehensive site evaluations, investigator selection, and compliance reviews, ensuring that all aspects of the experiment are meticulously considered.
Expert opinions highlight that effective viability evaluations not only enhance success rates but also foster trust among stakeholders, ultimately leading to more efficient and impactful medical research.

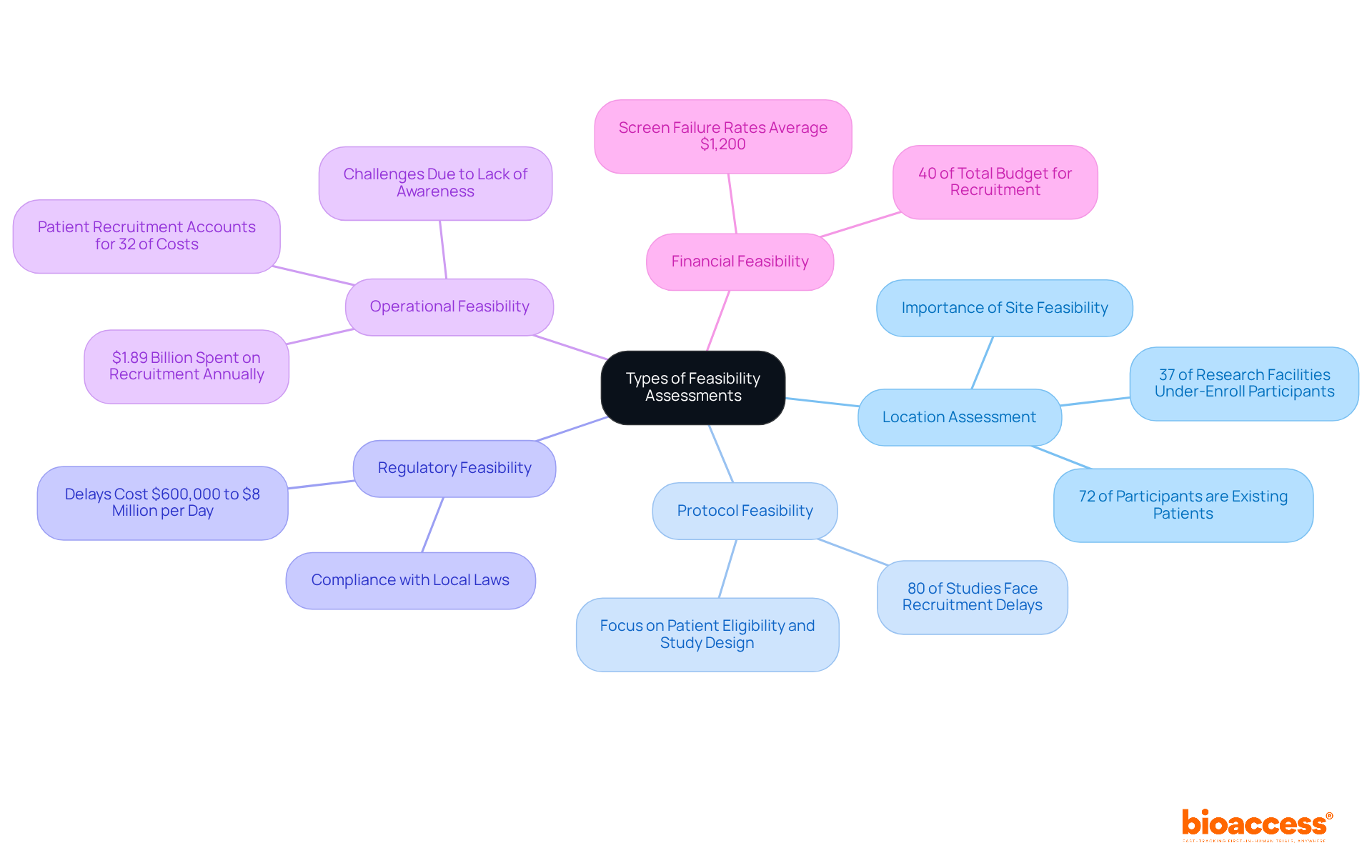
Viability evaluations are essential in medical research, guaranteeing that studies are organized for success. The following types of feasibility assessments are commonly conducted:
Location Assessment: This evaluation examines the medical facility's capabilities, including the qualifications of the personnel, availability of essential equipment, and previous experience with comparable studies. A well-equipped location can greatly improve recruitment and retention rates, acknowledging that 37% of research facilities under-enroll participants. Significantly, 72% of participants in the study are already patients at a location, emphasizing the importance of site feasibility in recruitment strategies.
Protocol Feasibility: This type assesses the practicality of the study protocol, focusing on patient eligibility criteria, study duration, and overall design. A well-organized protocol is crucial, as roughly 80% of medical studies encounter delays or shutdowns because of recruitment problems.
Regulatory Feasibility: This assessment examines the regulatory landscape to ensure compliance with local laws and guidelines, which can vary widely across regions. Understanding these regulations is vital for avoiding costly delays, which can range from $600,000 to $8 million for each day of delay. With bioaccess®'s expertise in navigating complex regulatory requirements, startups can accelerate their approval processes and mitigate risks associated with regulatory challenges.
Operational Feasibility: This focuses on logistical aspects such as recruitment strategies, patient access, and resource allocation. Effective operational planning is essential, particularly given that patient recruitment represents around 32% of research costs, totaling nearly $1.89 billion each year. Difficulties in recruiting patients frequently arise from a lack of awareness among possible participants regarding clinical studies, making effective communication vital.
Financial Feasibility: This analysis examines the budgetary implications of the experiment, ensuring that funding is secured and that costs align with expected outcomes. Considering that screen failure rates can average $1,200 per incident, a comprehensive financial evaluation can help reduce unforeseen costs and highlight the significance of meticulous financial planning.
By performing these evaluations, research teams can recognize possible obstacles early, optimize procedures, and improve the chances of favorable study results in the feasibility clinical trial. Insights from research specialists further emphasize the importance of these evaluations in achieving efficient and effective studies, particularly in the context of bioaccess®'s comprehensive management services for medical device studies, including accelerated research services tailored for early-feasibility, first-in-human, pilot, pivotal, and post-market follow-up investigations.
To conduct a comprehensive feasibility assessment, follow these essential steps:
Gather Preliminary Data: Collect relevant data on the target population, including demographics, disease prevalence, and potential recruitment sources. This foundational information is crucial for assessing patient availability and recruitment potential.
Evaluate Facility Capabilities: Assess the clinical facility's infrastructure, focusing on staff expertise, available equipment, and previous trial experience. Performing on-location visits can offer direct confirmation of these capabilities, ensuring the location is well-equipped to manage the study.
Review the Study Protocol: Analyze the study protocol for clarity and practicality. Ensure that the eligibility criteria are realistic and that the study design aligns with the site’s capabilities, which is vital for successful execution.
Engage Stakeholders: Involve key stakeholders—such as investigators, regulatory bodies, and ethics committees—early in the process. Their insights can help identify potential concerns and streamline the approval process, fostering a collaborative environment.
Analyze Regulatory Requirements: Review local regulations and guidelines to ensure compliance. Consulting with regulatory experts or legal advisors can help navigate complex requirements and avoid potential pitfalls, particularly in the context of Latin America where regulations can vary significantly by country.
Compile Findings: Document all findings in a feasibility report, highlighting strengths, weaknesses, and actionable recommendations. This report acts as an essential reference for decision-making, directing the subsequent steps in the medical research process.
Choose a Principal Investigator (PI): Identify and select a qualified principal investigator who has experience in conducting clinical studies and is familiar with the local regulatory environment. The principal investigator plays an essential role in the success of the study and should be involved early in the feasibility evaluation.
Review Study Documents: Ensure that all study documents comply with local requirements and are ready for submission to regulatory bodies. This step is crucial for enabling a seamless approval procedure and guaranteeing that the experiment can begin without interruptions.

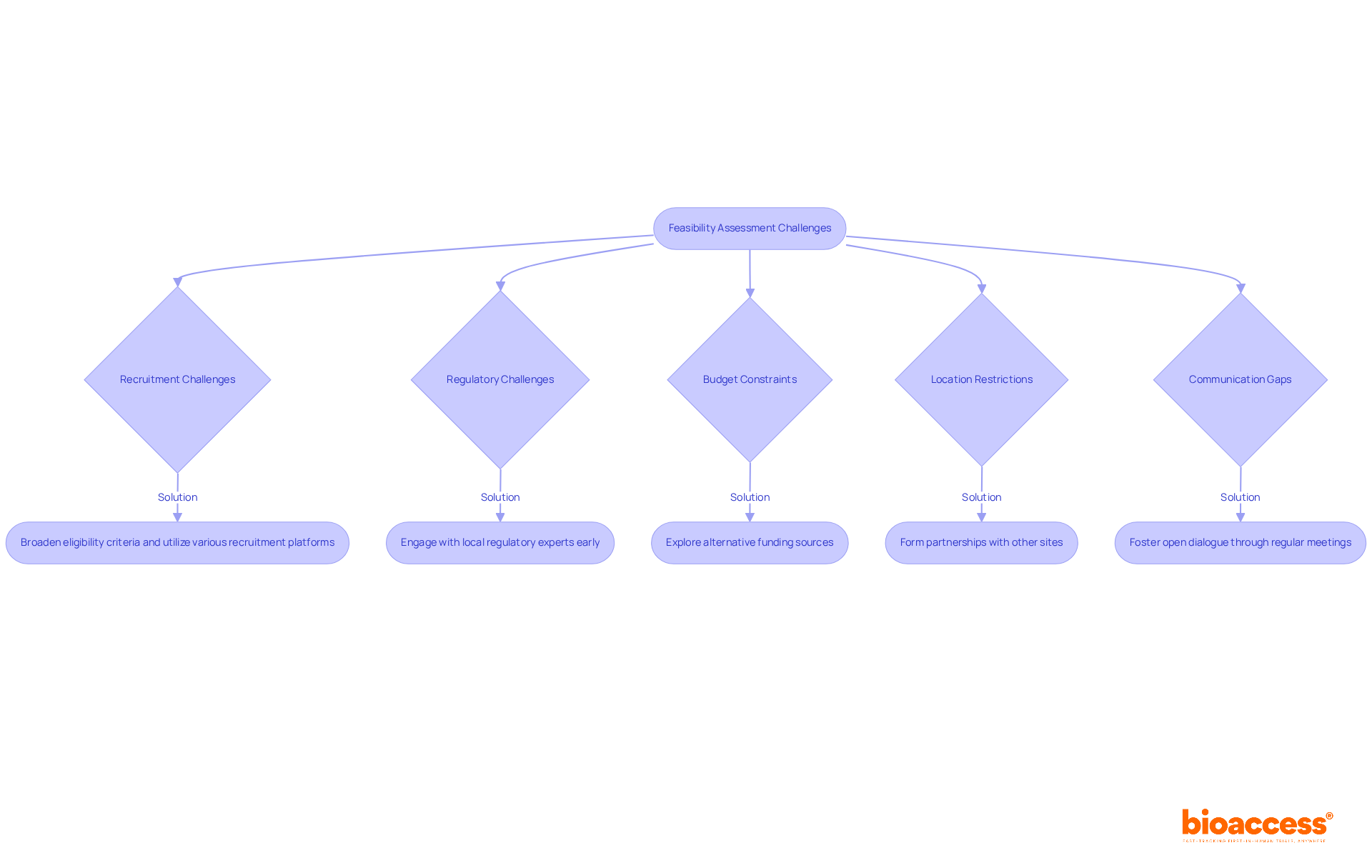
In medical studies, feasibility clinical trial evaluations often encounter numerous obstacles. Here are common issues along with suggested solutions:
Recruitment Challenges: Recruitment remains a significant hurdle, with research indicating that approximately 80% of medical studies fail to meet their patient recruitment goals within established timelines. In fact, 8 out of 10 studies struggle to enlist sufficient patients, and 70% of individuals eligible for a clinical study in the United States live more than 2 hours away from a research center. To address this, consider broadening eligibility criteria and utilizing various recruitment platforms to enhance patient access. Engaging with patient organizations and leveraging social media can also increase awareness and interest in participation. Notably, GlobalCare Clinical Trials, in partnership with bioaccess™, has achieved over a 50% reduction in recruitment time while maintaining a 95% retention rate in their studies in Colombia, exemplifying effective strategies to overcome these challenges.
Regulatory Challenges: Navigating complex regulatory environments can delay study initiation. To streamline this process, engage with local regulatory experts early to ensure compliance and expedite approvals. Understanding the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is essential as it oversees medical device regulations and classifications, functioning as a Level 4 health authority by PAHO/WHO. This proactive approach can significantly reduce the time to ethical clearance, which can be accomplished in as little as 4-6 weeks in regions like Latin America.
Budget Constraints: Financial feasibility is frequently a concern, with recruitment challenges potentially costing the pharmaceutical industry millions in delays. Recruitment difficulties can lead to study postponements, inflating development budgets, and are estimated to cost the pharmaceutical industry between USD 600,000 and 8 million per day of delay. Explore alternative funding sources, such as grants or partnerships, and consider adjusting the study design to minimize costs without compromising quality. Utilizing technology for remote participation can also help reduce logistical expenses.
Location Restrictions: If a location lacks specific capabilities, consider forming partnerships with other sites or investing in necessary resources to enhance their capacity. Investigator research centers often face geographical limitations in recruiting volunteers, making collaboration essential. This collaboration can bolster the overall infrastructure and support for the study, ensuring that all locations are adequately prepared to meet study requirements. For instance, ReGelTec's Early Feasibility Study on HYDRAFIL™ in Colombia successfully treated eleven patients with degenerative disc disease, demonstrating the effectiveness of well-coordinated site management and resource allocation.
Communication Gaps: Effective communication among all stakeholders is vital for promptly addressing concerns. Trust is paramount in patient recruitment for clinical studies, as individuals may hesitate to participate due to worries about data privacy and the study process. Foster open dialogue through regular meetings and updates to maintain alignment and transparency. This collaborative approach can help reduce misunderstandings and enhance the overall effectiveness of the feasibility evaluation process.
By anticipating these challenges and implementing proactive solutions, researchers can significantly improve the process of feasibility clinical trial assessment, ultimately increasing the likelihood of successful trial outcomes.
Conducting a feasibility clinical trial assessment is fundamental to ensuring the success of medical research endeavors. This process not only identifies potential hurdles but also equips researchers with the insights necessary to make informed decisions about study execution. By thoroughly evaluating regulatory requirements, patient demographics, site capabilities, and logistical considerations, researchers can significantly enhance the likelihood of a trial's success.
Throughout the article, key insights into the various types of feasibility assessments have been discussed, including:
Each type plays a vital role in addressing the complexities of clinical trials, particularly in the Medtech and Biopharma sectors, where the stakes are high and the costs of failure can be astronomical. The emphasis on proactive planning, stakeholder engagement, and effective communication stands out as crucial strategies for overcoming common challenges such as recruitment difficulties and regulatory hurdles.
Ultimately, the importance of mastering feasibility clinical trial assessments cannot be overstated. By adopting a comprehensive approach and leveraging expert insights, researchers can navigate the intricacies of clinical trials more effectively. This not only leads to more efficient studies but also fosters trust among stakeholders, paving the way for impactful advancements in medical research. Engaging with services like those offered by bioaccess® can further streamline this process, ensuring that studies are designed for success from the outset.
What is a feasibility clinical trial?
A feasibility clinical trial is the evaluation of both the practicality and likelihood of successfully conducting a clinical study. It involves assessing regulatory requirements, patient demographics, site capabilities, and logistical considerations.
Why is a thorough feasibility clinical trial evaluation important?
A thorough feasibility clinical trial evaluation is essential for identifying potential obstacles and ensuring that studies can be conducted ethically and efficiently. It helps researchers make informed decisions about whether to proceed with the study or modify its design.
What challenges do medical studies face regarding enrollment?
Approximately 80% of medical studies fail to meet initial enrollment targets, which can lead to recruitment delays that cost drug development firms up to $8 million daily.
In which sectors are successful feasibility clinical trials particularly critical?
Successful feasibility clinical trials are particularly critical in the Medtech and Biopharma sectors, where the complexity of studies has increased significantly over the years.
What management services does bioaccess® offer for feasibility clinical trials?
bioaccess® offers extensive management services for feasibility clinical trials, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), specifically tailored for the Latin American market.
What methodologies are employed in feasibility studies?
The methodologies employed in feasibility studies include comprehensive site evaluations, investigator selection, and compliance reviews, ensuring all aspects of the experiment are meticulously considered.
How do effective viability evaluations impact medical research?
Effective viability evaluations enhance success rates and foster trust among stakeholders, ultimately leading to more efficient and impactful medical research.