


Navigating the complex landscape of gene therapy trials in Montenegro offers significant opportunities and challenges for researchers. As genetic intervention emerges as a groundbreaking approach to treating diseases, grasping the regulatory framework governing these trials is crucial for success.
How can researchers effectively align their studies with evolving regulations while ensuring compliance and participant safety?
This guide explores the intricacies of gene therapy trial regulation in Montenegro, providing insights and strategies to bolster clinical research efforts in this rapidly advancing field.
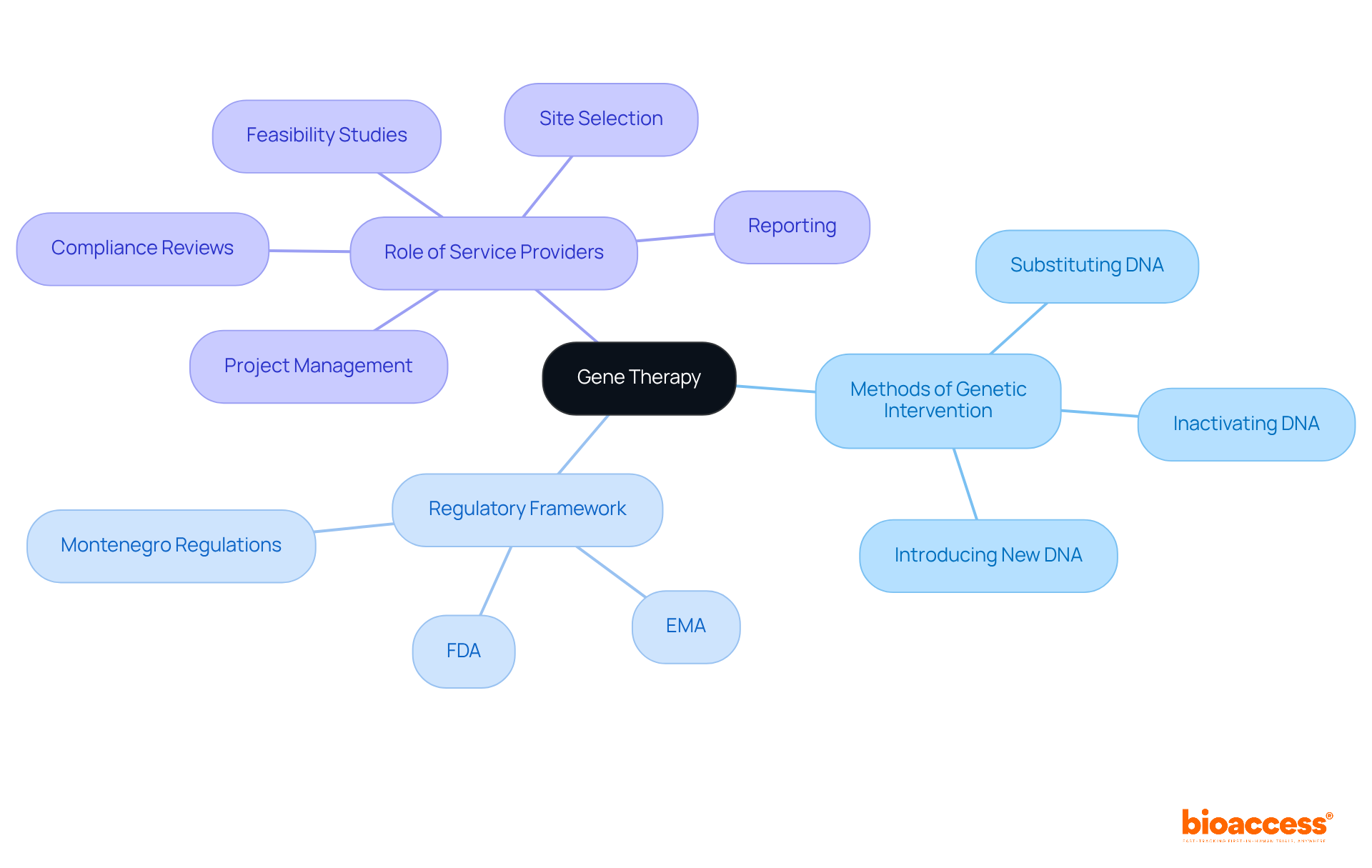
Genetic intervention stands as a groundbreaking method that modifies an individual's DNA to tackle or prevent illness. This innovative approach can involve:
The regulatory framework governing genetic treatment varies by region, typically incorporating directives from organizations such as the FDA in the United States and the EMA in Europe. In Montenegro, the regulatory landscape is evolving, aligning with EU standards to ensure safety and efficacy in gene therapy trial regulation in Montenegro.
Companies like bioaccess play a crucial role in this landscape by providing comprehensive clinical study management services. Their offerings include:
Understanding these frameworks and the support provided by knowledgeable service providers is essential for researchers aiming to navigate the complexities of clinical studies effectively. How can your organization leverage these insights to enhance your clinical research efforts?
In summary, collaboration with experienced partners like bioaccess is vital for advancing gene therapy studies. By aligning with established regulatory standards and utilizing expert services, researchers can ensure their projects comply with gene therapy trial regulation in Montenegro and are positioned for success. The next steps involve engaging with these service providers to explore tailored solutions that meet your specific research needs.
Montenegro's regulatory environment for genetic research studies stands out due to its strong adherence to EU standards, showcasing a commitment to bioethics and clinical research governance. The Institute for Medicines and Medical Devices of Montenegro plays a pivotal role in overseeing the approval process for clinical studies, particularly those related to gene therapy trial regulation in Montenegro. Researchers must submit detailed applications that include:
to obtain the necessary approvals. This thorough approach not only ensures compliance with Good Clinical Practice (GCP) standards but also facilitates the swift initiation of studies.
Notably, the average time for clinical study approval in Montenegro is remarkably efficient, often completed within a few weeks. This efficiency is a significant advantage for researchers eager to expedite their studies. Furthermore, the ethical framework governing these studies is meticulously designed to protect participant rights and safety, ensuring that all clinical research is conducted transparently and responsibly. As Montenegro continues to enhance its regulatory structure, the focus on gene therapy trial regulation in Montenegro, along with bioethics and adherence to EU standards, will remain central to its clinical research oversight, fostering an environment conducive to groundbreaking genetic research.

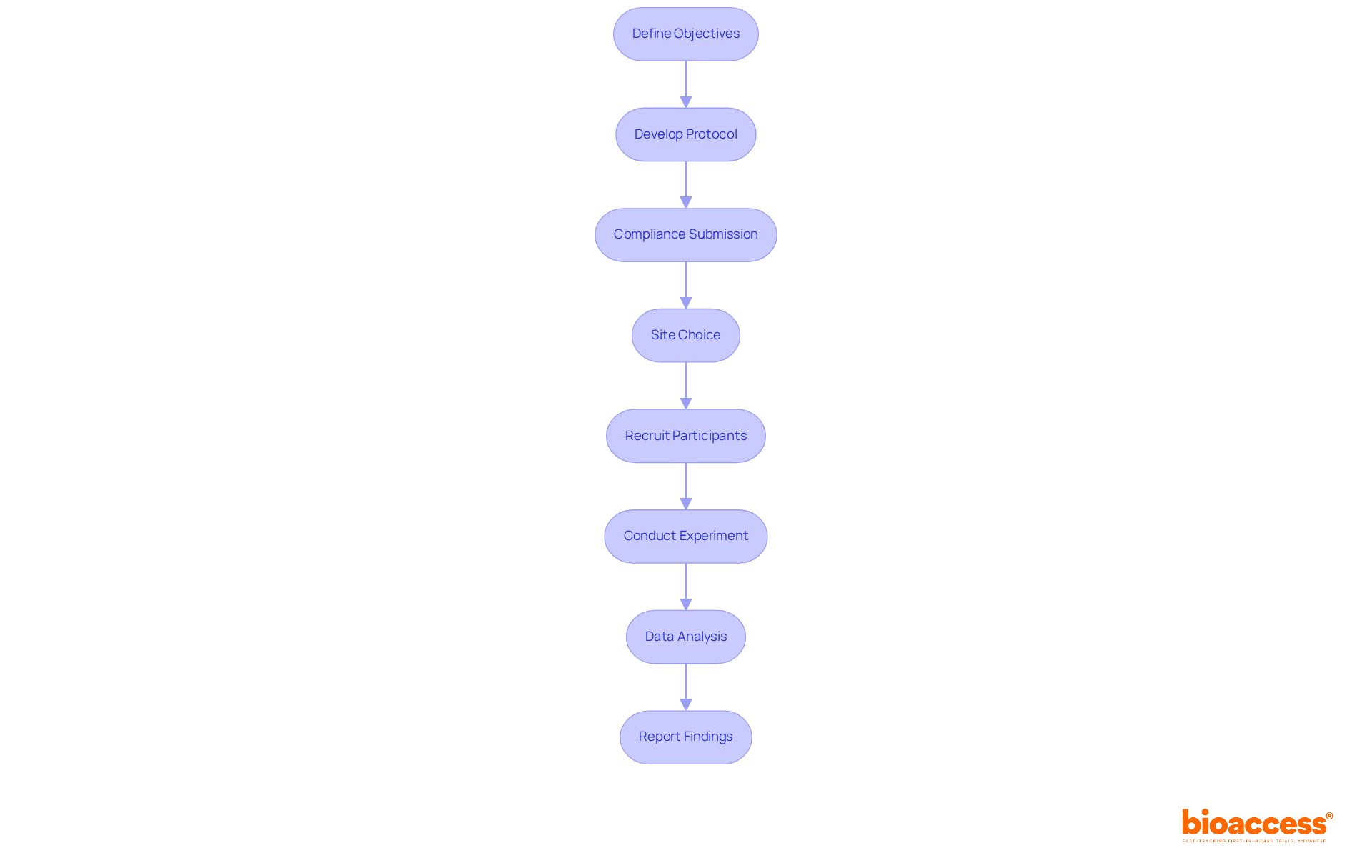
Define Objectives: Clearly articulate the goals of the gene therapy study. Specify the target population and anticipated outcomes to guide the study's direction effectively.
Develop Protocol: Construct a comprehensive study protocol that encompasses methodology, participant eligibility criteria, and safety measures. This ensures a robust research design that can withstand scrutiny.
Compliance Submission: Compile and submit all necessary documentation to oversight authorities, adhering to both local and EU regulations. Notably, in Montenegro, the average duration for submission approval under the gene therapy trial regulation in Montenegro is remarkably efficient, often finalized within a few weeks, enabling faster study initiation.
Site Choice: Identify and select clinical study locations equipped with the necessary infrastructure and expertise. This is crucial for effectively conducting genetic intervention studies while ensuring compliance with regulatory standards.
Recruit Participants: Implement targeted recruitment strategies to enroll eligible participants. It is essential to ensure that informed consent is thoroughly obtained and documented, safeguarding participant rights.
Conduct Experiment: Execute the study in strict accordance with the approved protocol. Continuous monitoring for safety and efficacy throughout the study duration is vital to uphold research integrity.
Data Analysis: Systematically examine the collected information to assess the results of the genetic intervention. Utilize suitable statistical techniques to ensure the validity of the findings, reinforcing the study's credibility.
Report Findings: Prepare and submit thorough reports to oversight bodies and stakeholders. These reports should outline study results, methodologies, and any negative occurrences faced during the research, fostering transparency and trust.
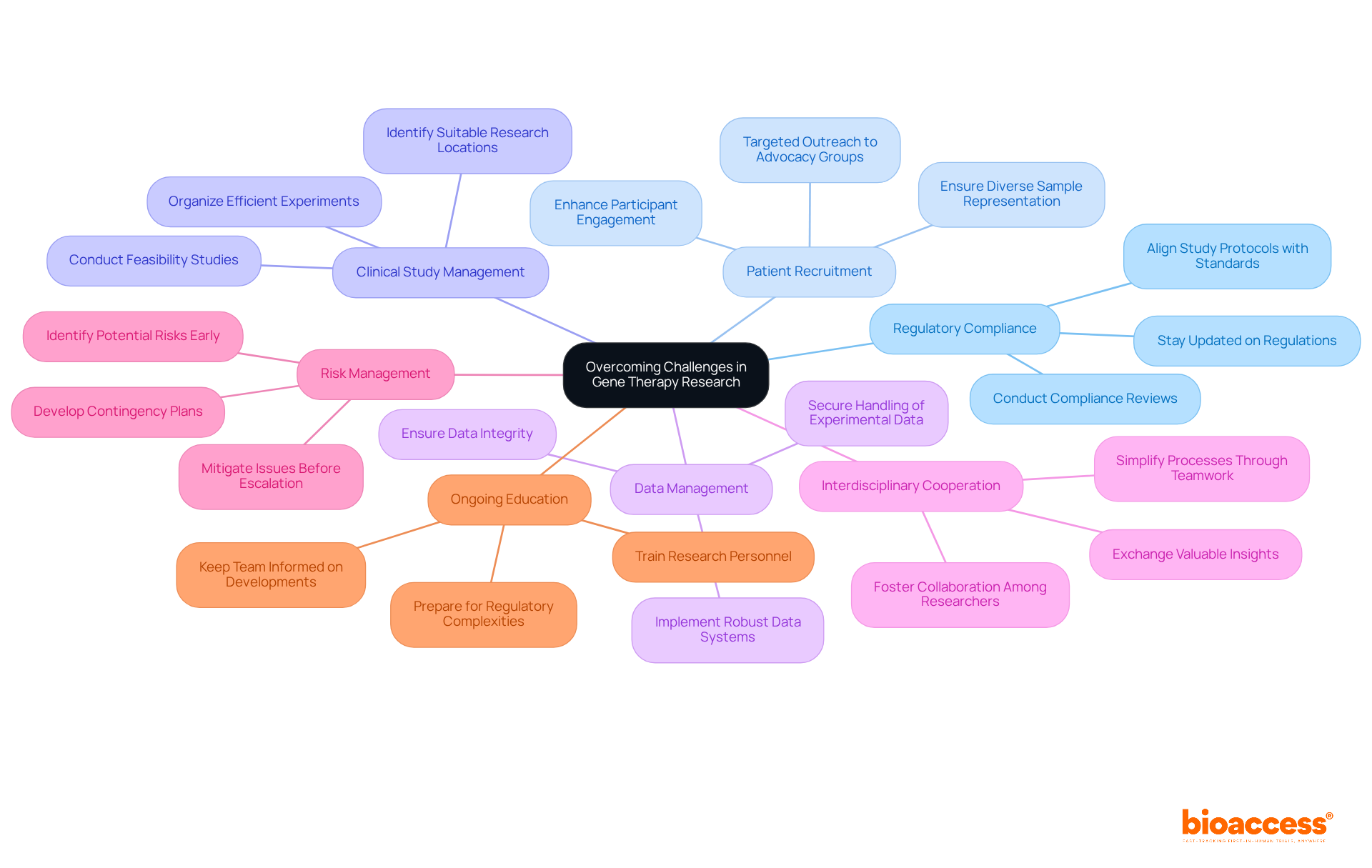
Regulatory compliance is a cornerstone in the context of gene therapy trial regulation in Montenegro. It's essential to stay abreast of the gene therapy trial regulation in Montenegro and ensure that all study protocols align with current standards. This vigilance is crucial to avoid delays that could jeopardize the study's timeline. Equally important are effective patient recruitment strategies; targeted outreach to patient advocacy groups can significantly enhance participant engagement, ensuring a diverse and representative sample.
At bioaccess, we offer comprehensive clinical study management services that directly address these challenges. Our feasibility studies play a pivotal role in identifying suitable research locations and lead investigators, ensuring that experiments are organized efficiently. We conduct meticulous compliance reviews of study documents to align with country requirements, which is vital for ensuring adherence to gene therapy trial regulation in Montenegro and steering clear of regulatory pitfalls. Our expertise in setting up examinations, including acquiring necessary import permits and nationalization of investigational devices, streamlines the approval process with ethics committees and health ministries.
Implementing robust data management systems is critical for the precise and secure handling of experimental data, maintaining integrity throughout the research process. Interdisciplinary cooperation among researchers, clinicians, and compliance specialists simplifies processes and fosters the exchange of valuable insights, ultimately enhancing study efficiency.
Proactively identifying potential risks early in the testing process enables the development of effective contingency plans, mitigating issues before they escalate. Ongoing education and training for research personnel are essential to keep them informed about the latest developments in gene therapy and the gene therapy trial regulation in Montenegro, ensuring that the team is well-prepared to navigate the complexities of clinical studies. Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics, spearheads our efforts to ensure compliance and best practices throughout the trial lifecycle.
The exploration of gene therapy in Montenegro underscores the critical importance of adhering to established regulatory frameworks while embracing innovative approaches to genetic intervention. Understanding the evolving landscape of gene therapy trial regulations enables researchers to navigate the complexities involved in bringing groundbreaking treatments to fruition effectively.
Key insights from this guide highlight the necessity of collaboration with experienced service providers, such as bioaccess, to facilitate compliance and streamline the clinical study process. From defining objectives and developing protocols to ensuring participant safety and executing robust data management systems, each step is vital for the success of gene therapy trials. Moreover, Montenegro's regulatory environment, marked by its efficiency and commitment to bioethics, offers a favorable backdrop for advancing research in this transformative field.
Ultimately, the future of gene therapy in Montenegro hinges on proactive engagement with regulatory standards and a steadfast commitment to ethical practices. Researchers are encouraged to leverage the insights provided in this guide to enhance their clinical research efforts, ensuring they are well-equipped to overcome challenges and contribute to the advancement of gene therapy. By fostering collaboration and maintaining a focus on compliance, the potential for successful gene therapy outcomes can be significantly amplified, paving the way for innovative treatments that can transform patient care.
What is gene therapy?
Gene therapy is a groundbreaking method that modifies an individual's DNA to tackle or prevent illness. It can involve substituting a defective segment of DNA, inactivating a malfunctioning segment, or introducing a new segment to combat disease effectively.
How does the regulatory framework for gene therapy vary?
The regulatory framework for gene therapy varies by region, typically incorporating directives from organizations such as the FDA in the United States and the EMA in Europe. In Montenegro, the regulatory landscape is evolving to align with EU standards to ensure safety and efficacy in gene therapy trial regulation.
What role do companies like bioaccess play in gene therapy?
Companies like bioaccess provide comprehensive clinical study management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
Why is understanding the regulatory framework important for researchers?
Understanding the regulatory framework and the support provided by knowledgeable service providers is essential for researchers to navigate the complexities of clinical studies effectively.
What is recommended for advancing gene therapy studies?
Collaboration with experienced partners like bioaccess is vital for advancing gene therapy studies. By aligning with established regulatory standards and utilizing expert services, researchers can ensure their projects comply with gene therapy trial regulation in Montenegro and are positioned for success.
What are the next steps for organizations interested in gene therapy research?
The next steps involve engaging with service providers to explore tailored solutions that meet specific research needs in gene therapy.