


This article underscores the critical importance of mastering Good Clinical Practice (GCP) compliance in clinical trials, highlighting its essential role in safeguarding participant welfare and ensuring data integrity. It elaborates on key principles of GCP, including:
All of which are vital for enhancing the credibility of clinical research and securing regulatory approval. By understanding and implementing these principles, stakeholders can significantly improve the quality and reliability of clinical trials, ultimately advancing the field of medical research.
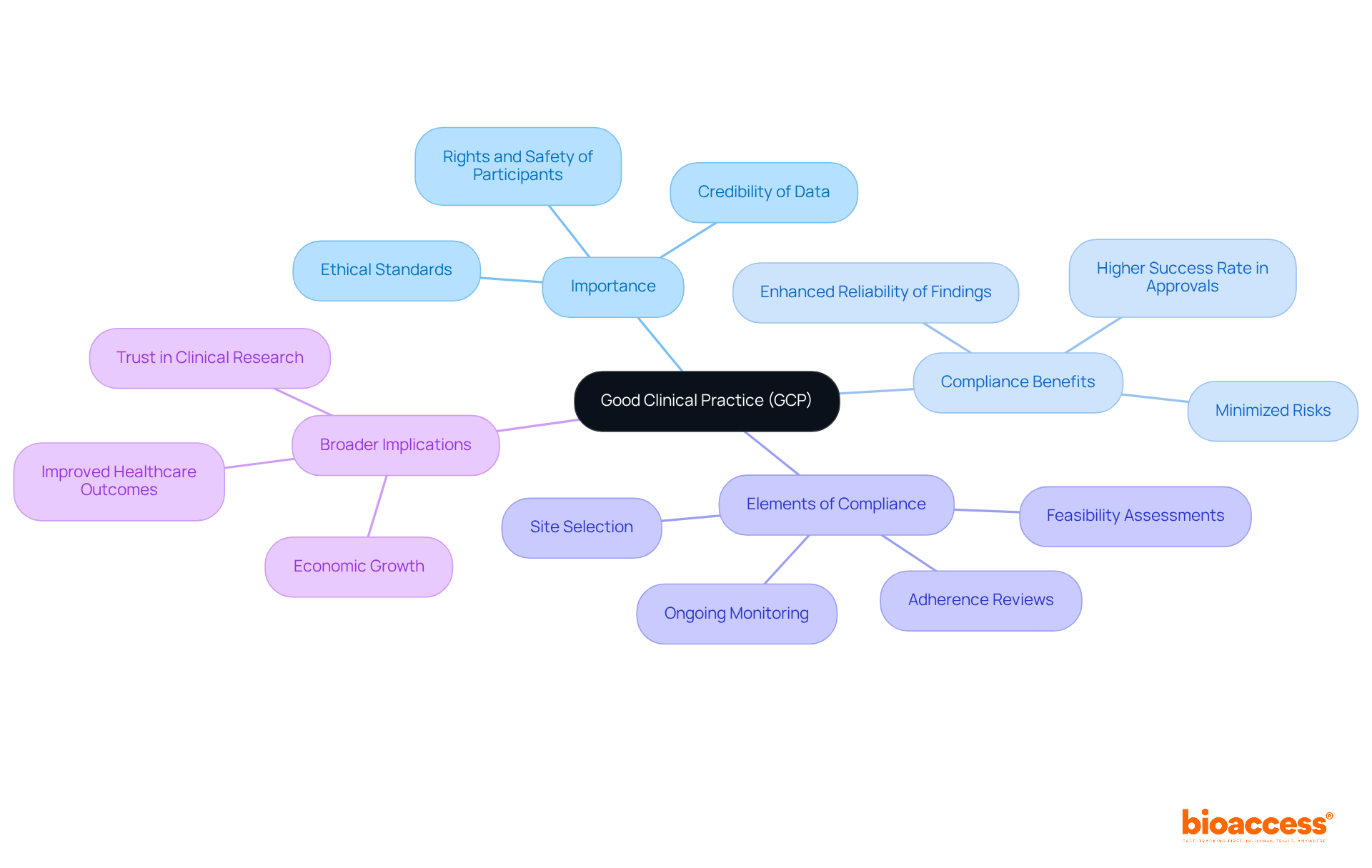
Good Clinical Practice (GCP) stands as the cornerstone of ethical and scientifically rigorous clinical trials, safeguarding the safety and rights of participants while bolstering the reliability of research outcomes.
In the ever-evolving landscape of clinical research, mastering GCP compliance is now more critical than ever for researchers seeking to navigate regulatory complexities and secure approval for their studies.
However, researchers face numerous challenges, including inadequate training and documentation issues.
This raises an important question: how can researchers effectively implement GCP standards to foster trust and credibility in their findings?
Good Clinical Practice (GCP) stands as an internationally recognized quality standard, established by the International Council for Harmonisation (ICH), that guides regulatory authorities worldwide. This standard encompasses ethical and scientific quality benchmarks essential for the design, conduct, recording, and reporting of clinical studies involving human subjects. The adherence to good clinical practice compliance is paramount; it safeguards the rights, safety, and welfare of participants while ensuring the credibility and precision of the data produced.
By ensuring good clinical practice compliance, researchers not only enhance the reliability of their findings but also fulfill critical requirements for obtaining regulatory approval and advancing medical knowledge. Statistics indicate that good clinical practice compliance significantly influences clinical research outcomes, as studies show that projects adhering to these guidelines achieve a higher success rate in securing approval from regulatory agencies.
Furthermore, bioaccess's comprehensive clinical research management services—including feasibility assessments, site selection, adherence reviews, setup, import permits, project management, and reporting on both serious and non-serious adverse events—underscore a strong commitment to GCP adherence.
Case studies highlight that good clinical practice compliance is vital for protecting study participants, as it mandates ongoing monitoring and adherence to established protocols, thereby minimizing risks and ensuring ethical conduct throughout the research process. This commitment not only builds trust in clinical research but also stimulates local economies through job creation, economic growth, and improved healthcare outcomes.
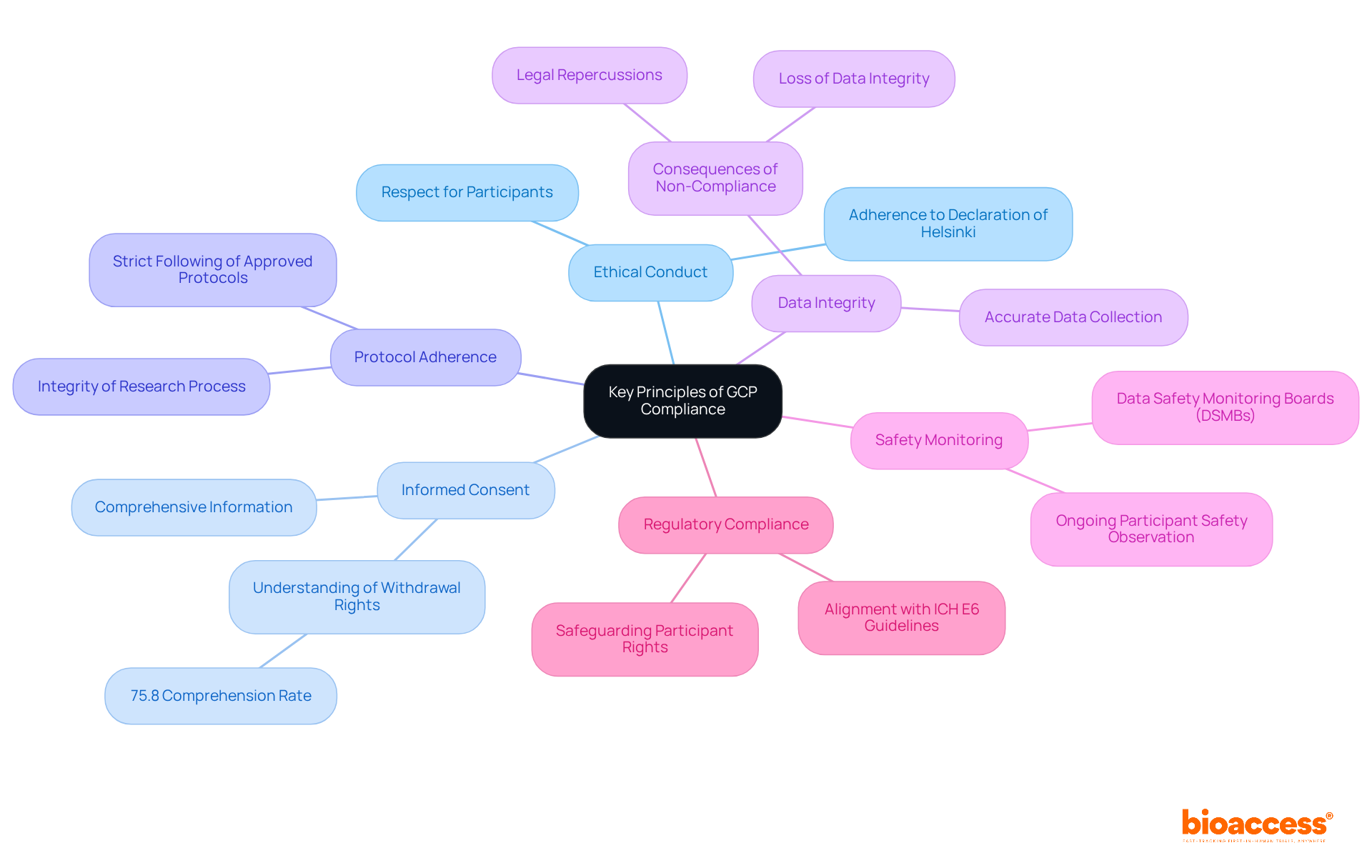
The key principles of good clinical practice compliance are foundational to conducting ethical and scientifically sound clinical trials.
Ethical Conduct: Trials must adhere to ethical principles rooted in the Declaration of Helsinki, which emphasizes respect for participants and their rights.
Informed Consent: Participants should receive comprehensive information about the study, ensuring they provide voluntary and informed consent prior to enrollment. Despite advancements, research shows that only 75.8% of individuals completely comprehend their right to opt out of experiments, emphasizing the necessity for clearer communication.
Protocol Adherence: Each experiment must strictly follow a pre-approved protocol detailing the project's objectives, design, methodology, and statistical considerations. This adherence is crucial for maintaining the integrity of the research process.
Data Integrity: The collection, recording, and reporting of data must be accurate and reliable. Non-compliance can lead to significant consequences, including loss of data integrity and legal repercussions, which can undermine public trust in clinical research.
Safety Monitoring: Ongoing observation of participant safety is crucial throughout the study. This includes proactive reporting mechanisms and the establishment of Data Safety Monitoring Boards (DSMBs) to oversee safety protocols.
Regulatory Compliance: All research activities must align with applicable regulatory requirements and guidelines, such as those set forth by the ICH E6. Compliance with these regulations, particularly good clinical practice compliance, not only safeguards participant rights but also enhances the credibility of the research findings.
In addition to these principles, extensive clinical study management services, such as feasibility studies, site selection, adherence reviews, study setup, import permits, project management, and reporting offered by bioaccess, play a vital role in ensuring good clinical practice compliance. Grasping and applying these principles is crucial for ensuring adherence and the overall success of clinical studies, fostering trust among stakeholders and the communities involved.
To effectively implement Good Clinical Practice (GCP) standards in clinical trials, it is essential to consider the following steps:
Develop a Comprehensive Protocol: Create a thorough and transparent research protocol that adheres to GCP guidelines, ensuring all facets of the investigation are addressed.
Train Your Team: Conduct thorough training sessions for all team members on GCP principles and the specific requirements of the study. Notably, only 13% of clinical research professionals (CRPs) completely adhered to training protocols in recent investigations, underscoring the necessity for engaging and effective training methods.
Establish a Quality Management System: Implement a robust system for monitoring good clinical practice compliance throughout the study, including regular audits and reviews to identify areas for improvement.
Ensure Informed Consent: Develop clear and concise informed consent documents, ensuring participants fully understand their rights and the study's purpose. Research indicates that patients who do not read informed consent documents are nearly three times more likely to refuse participation in the study.
Document Everything: Maintain meticulous records of all trial activities, including participant interactions, data collection, and adverse events. Proper documentation is essential for ensuring data integrity and adherence to regulatory standards.
Engage with Regulatory Authorities: Foster open communication with regulatory bodies to ensure ongoing adherence and promptly address any concerns. This proactive approach can significantly enhance the credibility of your research.
Moreover, utilizing bioaccess's extensive clinical research management services—including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—can greatly improve the quality and credibility of your research. By adhering to these procedures and employing professional services, researchers can effectively incorporate GCP standards into their clinical studies, thereby enhancing the quality and credibility of their research outcomes. Successful case examples demonstrate that adherence to GCP not only bolsters trial integrity but also increases the likelihood of regulatory approval, ultimately benefiting patient safety and fostering medical innovation.

Challenges in GCP compliance can arise from various sources, including:
Lack of Training: Insufficient training can result in misunderstandings of GCP requirements, a significant concern as studies indicate that inadequate training contributes to regulatory failures.
Solution: Implement regular training sessions and refreshers for all staff involved in the trial. This approach not only enhances understanding but also fosters a culture of compliance.
Insufficient Documentation: Subpar record-keeping can undermine data integrity, with research indicating that documentation mistakes can vary from 2 to 2,784 errors per 10,000 fields. This emphasizes the essential requirement for careful record management.
Solution: Establish a robust documentation system that includes templates and checklists to ensure thorough record-keeping. This system should be regularly audited to maintain high standards.
Participant Recruitment Issues: Difficulty in recruiting participants can delay trials, often exacerbated by a lack of outreach to diverse populations.
Solution: Create a focused recruitment approach that involves reaching out to varied populations and transparent communication of the project's advantages. Offering incentives, such as transportation reimbursement, can also encourage participation.
Regulatory Changes: Modifications in regulations can create adherence challenges, particularly in a rapidly evolving landscape where over 499,000 studies are registered on ClinicalTrials.gov. This necessitates constant vigilance.
Solution: Stay informed about regulatory updates and adjust protocols and practices accordingly. Regularly scheduled assessments of adherence practices can help ensure alignment with current regulations.
By proactively addressing these challenges, researchers can enhance their GCP compliance and ensure the success of their clinical trials.

Good Clinical Practice (GCP) compliance stands as a cornerstone of ethical and scientifically sound clinical research, ensuring the safety, rights, and welfare of participants while enhancing the credibility of study results. The significance of adhering to GCP standards cannot be overstated; it fosters trust in clinical trials and plays a crucial role in advancing medical knowledge and achieving successful regulatory approvals.
Throughout this article, we have outlined key principles of GCP compliance, including:
Each of these elements contributes to the overall framework necessary for conducting responsible clinical trials. Furthermore, practical steps for implementing GCP standards have been discussed, such as:
Addressing common challenges like insufficient training and documentation issues underscores the importance of a proactive approach to GCP compliance.
Ultimately, the commitment to Good Clinical Practice is vital not just for the integrity of individual studies but for the broader landscape of clinical research. By prioritizing GCP adherence, researchers can significantly improve trial outcomes, enhance participant safety, and contribute to the collective goal of advancing healthcare. Embracing these practices is not merely a regulatory obligation; it is an ethical imperative that ensures the trust and safety of all involved in the clinical research process.
What is Good Clinical Practice (GCP)?
Good Clinical Practice (GCP) is an internationally recognized quality standard established by the International Council for Harmonisation (ICH) that provides guidelines for the ethical and scientific quality of clinical studies involving human subjects.
Why is GCP important?
GCP is important because it safeguards the rights, safety, and welfare of participants in clinical studies while ensuring the credibility and precision of the data produced. Compliance with GCP enhances the reliability of research findings and is critical for obtaining regulatory approval.
How does GCP compliance affect clinical research outcomes?
GCP compliance significantly influences clinical research outcomes, as studies adhering to these guidelines have a higher success rate in securing approval from regulatory agencies.
What services does bioaccess offer to support GCP adherence?
Bioaccess offers comprehensive clinical research management services, including feasibility assessments, site selection, adherence reviews, setup, import permits, project management, and reporting on both serious and non-serious adverse events, all aimed at ensuring GCP adherence.
How does GCP compliance protect study participants?
GCP compliance protects study participants by mandating ongoing monitoring and adherence to established protocols, which minimizes risks and ensures ethical conduct throughout the research process.
What are the broader benefits of GCP compliance in clinical research?
GCP compliance builds trust in clinical research, stimulates local economies through job creation and economic growth, and improves healthcare outcomes.