


The primary focus of this article is on strategies for managing heterogeneity in clinical trials, crucial for their success. It emphasizes the necessity of recognizing various types of heterogeneity—clinical, methodological, and statistical. To enhance the validity and applicability of trial outcomes across diverse patient populations, the article suggests approaches such as:
These strategies are essential for ensuring that clinical trials can effectively address the complexities of real-world patient demographics.
Understanding the complexities of heterogeneity in clinical trials is essential for researchers aiming to enhance the relevance and applicability of their findings. Variability among patient populations—shaped by factors such as age, gender, and health status—can significantly influence treatment outcomes. Therefore, it is imperative to adopt tailored strategies.
However, how can researchers effectively navigate these challenges to ensure their trials yield meaningful and generalizable results? This article delves into the intricacies of clinical trial heterogeneity and offers actionable strategies to master it, ultimately paving the way for successful research outcomes.
Heterogeneity in clinical studies indicates the variability in effects seen across different patient populations or environments. This variability is a result of the heterogeneity in patient traits—such as age, gender, ethnicity, and comorbidities—as well as variations in care protocols and the outcomes assessed. Understanding this heterogeneity is crucial, as it directly impacts the generalizability of trial outcomes and the effectiveness of treatments across various populations.
For example, a medication demonstrating considerable effectiveness in one group may yield differing outcomes in another, underscoring the necessity for customized strategies in research. Current analyses indicate that high levels of within-group heterogeneity can restrict the effectiveness of subgroup analyses, with the statistic for study variation nearing its maximum of 1, signaling substantial variation.
Real-world examples, such as the systematic review and meta-analysis conducted by Sabitova et al., illustrate the significant influence of geographical and demographic factors on study outcomes. In this review, 32% of participants reported high levels of job burnout, with variations shaped by specific countries. These insights emphasize the essential role of comprehending heterogeneity in enhancing the relevance and applicability of research findings in healthcare.
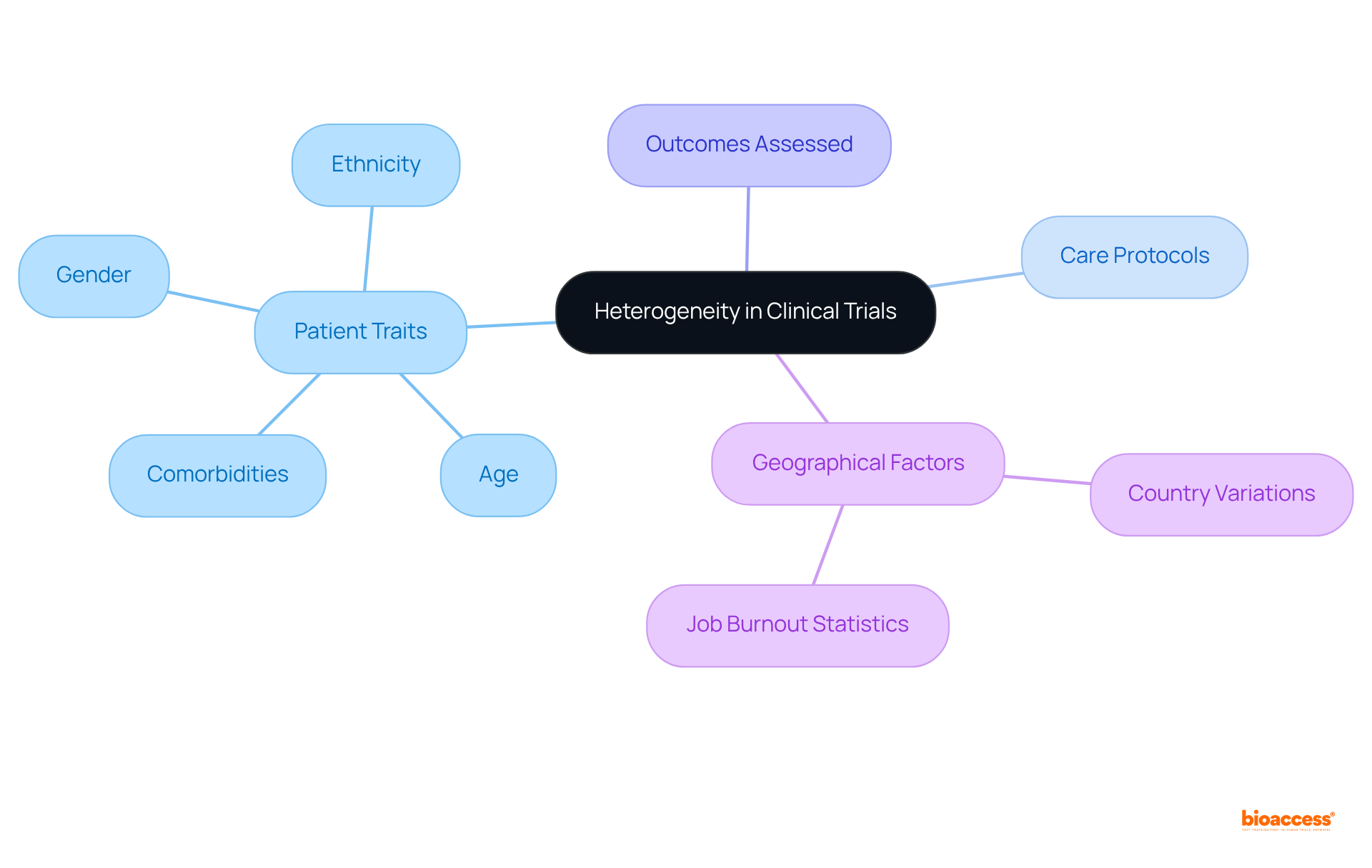
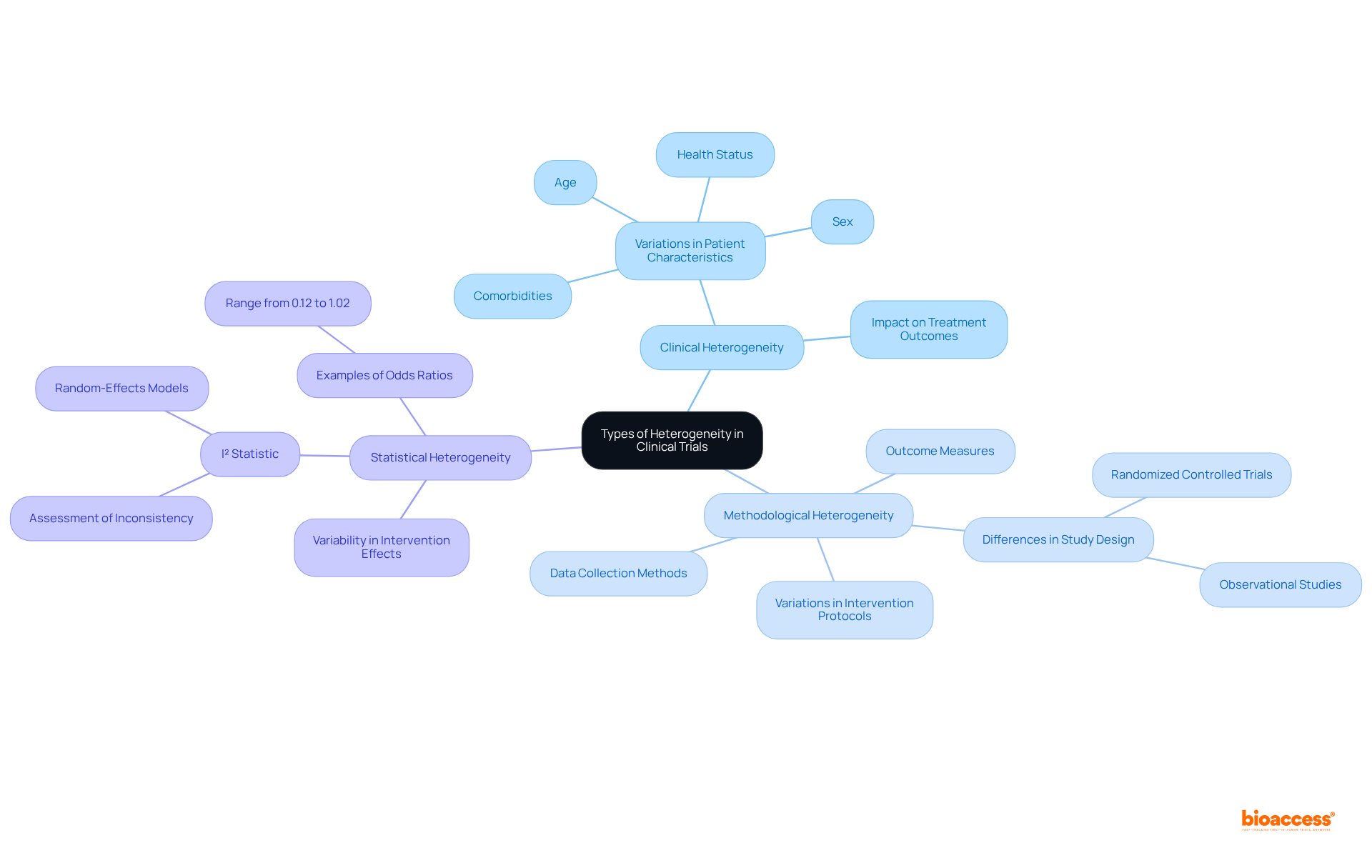
In clinical trials, understanding the different forms of heterogeneity is crucial for effective study design and analysis.
Clinical Heterogeneity: This refers to variations in patient characteristics, such as age, sex, comorbidities, and health status. For instance, a trial examining a new medication may include participants with differing baseline health conditions, which can significantly influence treatment outcomes. As Chalmers I aptly questions, 'Is there excessive variability in the cases for the review to be meaningful?' This underscores the necessity of recognizing heterogeneity in clinical study design.
This type of methodological heterogeneity encompasses differences in study design, incorporating variations in intervention protocols, outcome measures, and data collection methods. For example, one study might utilize a randomized controlled trial design while another employs an observational approach, leading to challenges in synthesizing results. A case study titled "Types of Heterogeneity" outlines these differences, emphasizing their significance in research planning.
Statistical Heterogeneity: This indicates variability in intervention effects across studies, often quantified using statistical methods. The I² statistic is commonly employed in meta-analyses to assess the degree of inconsistency among study results. High I² values suggest significant variability, prompting researchers to consider random-effects models to address this diversity. For instance, the odds ratios for medications to prevent cutaneous allergic reactions range from 0.12 to 1.02, illustrating the variability in treatment effects.
Identifying these types of heterogeneity early in the study design phase allows researchers to customize their methodologies efficiently. By accommodating the diverse responses expected from different patient groups, they can enhance the validity and reliability of their findings, ultimately leading to more robust conclusions.
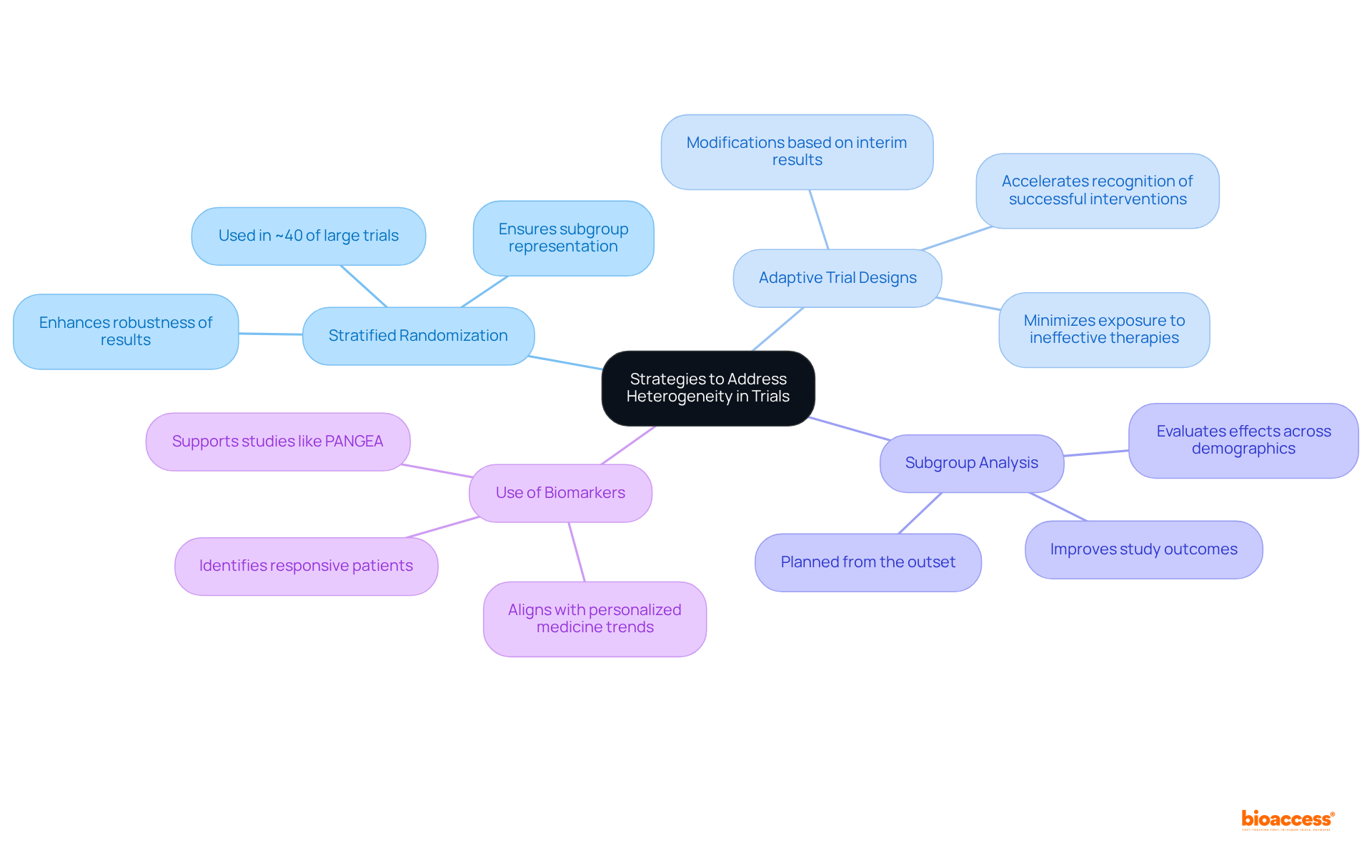
To effectively address the issue of heterogeneity in clinical trials, researchers should consider several key strategies.
Stratified Randomization stands out as a vital technique, ensuring that various subgroups are adequately represented in each intervention group by stratifying participants based on essential traits. Notably, approximately 40% of large clinical trials utilize this method to maintain group balance, thereby enhancing the robustness of results.
Adaptive Trial Designs are another crucial approach. These designs allow for modifications based on interim results, enabling researchers to respond to observed heterogeneity. This adaptability not only accelerates the recognition of successful interventions but also minimizes unnecessary exposure to ineffective therapies. The FDA has acknowledged the potential of adaptive designs, promoting a more patient-centric approach to drug development.
Subgroup Analysis should be planned from the outset to evaluate effects across diverse patient populations. This foresight can reveal critical insights into how different demographics respond to interventions, ultimately improving study outcomes.
Furthermore, the Use of Biomarkers is essential for identifying patients likely to respond positively to specific therapies. This precision enhances the study's effectiveness and aligns with the growing trend of personalized medicine, as evidenced by research like the PANGEA study, which assesses treatment algorithms based on molecular profiles.
By implementing these strategies, researchers can significantly mitigate the effects of heterogeneity, which will lead to improved study quality and more reliable results.
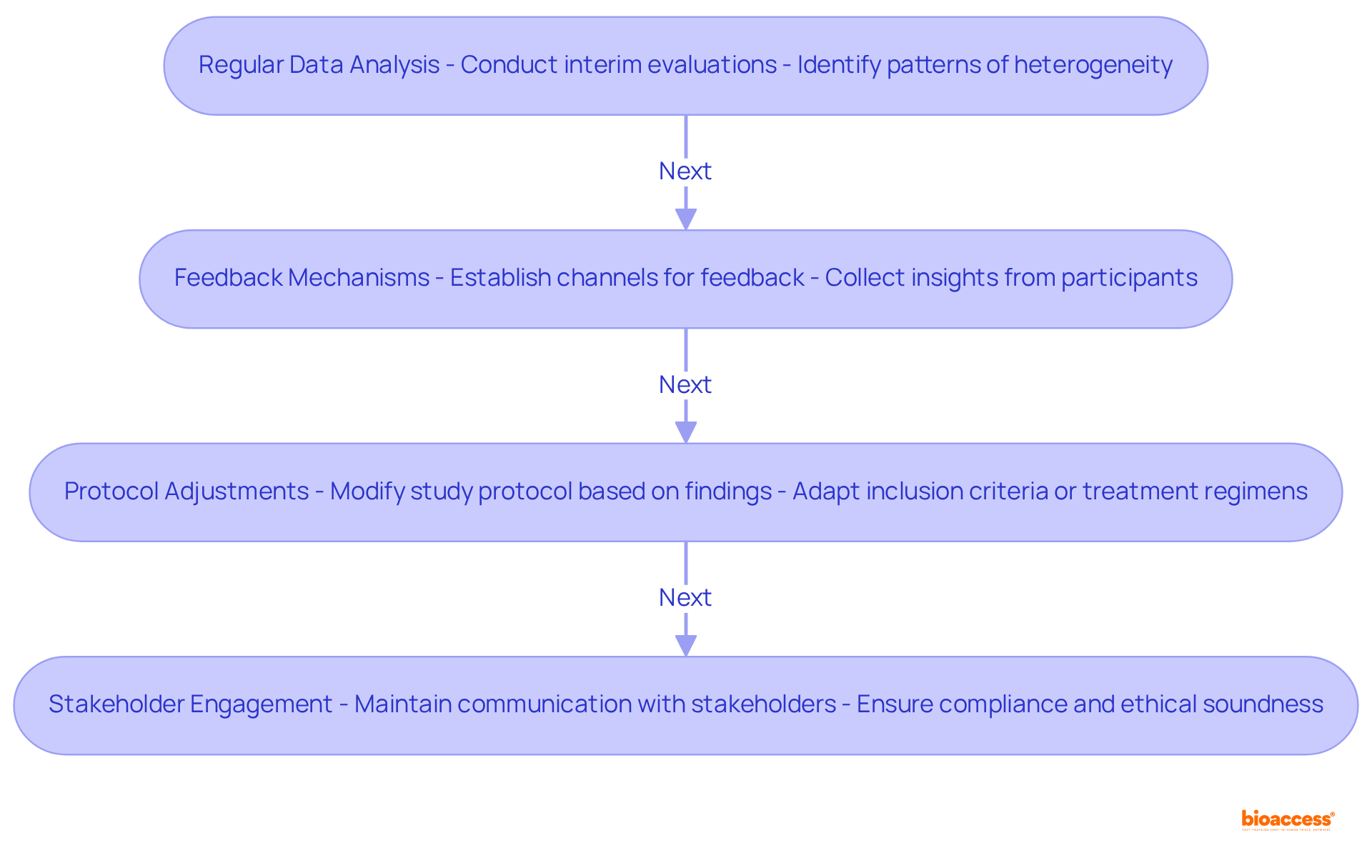
Observing variability during a clinical trial is crucial for guaranteeing the validity and relevance of findings. Key strategies include:
Regular Data Analysis: Conduct interim evaluations to assess effects across various subgroups, allowing for the identification of emerging patterns of heterogeneity. This proactive approach can reveal significant differences in responses that may not be apparent in initial analyses. For instance, with bioaccess®, ethical approvals are delivered in 4-6 weeks, enabling timely interim analyses that can inform ongoing strategies.
Feedback Mechanisms: Establish robust channels for feedback from clinical sites and participants. Collecting insights on responses to therapies and unforeseen variations can guide ongoing analyses and assist in improving study strategies. As James Merlino emphasizes, focusing on patient experience is crucial for success in today's competitive healthcare landscape, highlighting the importance of participant feedback.
Protocol Adjustments: Be prepared to modify the study protocol based on findings from interim analyses. This may involve modifying inclusion criteria or treatment regimens to better align with observed data, thereby enhancing the study's relevance and effectiveness. The ability to adapt quickly is vital, especially when enrollment is 50% faster than traditional markets, as seen in bioaccess®'s approach.
Stakeholder Engagement: Maintain transparent communication with stakeholders, including regulatory bodies and ethics committees. Ensuring that any adaptations are compliant and ethically sound is crucial for maintaining the integrity of the study. Including qualitative assessments regarding clinical variability can enhance conversations with stakeholders, as these assessments are frequently grounded in arguments about similarities or distinctions among studies.
By actively monitoring and adapting strategies throughout the trial, researchers can effectively manage heterogeneity, which ultimately enhances the robustness and credibility of their findings.
Understanding and managing heterogeneity in clinical trials is paramount for achieving meaningful and applicable results. The variability in patient responses, influenced by factors such as demographics and health conditions, underscores the necessity for tailored research strategies. By recognizing and addressing heterogeneity, researchers can enhance the generalizability of their findings, ultimately leading to improved treatment outcomes across diverse populations.
The article outlines several critical types of heterogeneity—clinical, methodological, and statistical—that researchers must consider during study design. It emphasizes strategies such as:
to effectively manage these variations. Additionally, ongoing monitoring and adaptation of trial strategies based on real-time data are essential for maintaining the integrity and relevance of the research.
In conclusion, the significance of addressing heterogeneity in clinical trials cannot be overstated. As the landscape of medical research evolves, employing best practices for managing variability will not only bolster the credibility of findings but also enhance patient care. Researchers are encouraged to prioritize these strategies, ensuring that clinical trials are equipped to deliver insights that are both reliable and applicable in real-world settings.
What does heterogeneity in clinical trials refer to?
Heterogeneity in clinical trials refers to the variability in effects observed across different patient populations or environments, influenced by factors such as patient traits, care protocols, and outcome assessments.
Why is understanding heterogeneity important in clinical trials?
Understanding heterogeneity is crucial because it impacts the generalizability of trial outcomes and the effectiveness of treatments across various populations.
How can patient traits contribute to heterogeneity in clinical trials?
Patient traits such as age, gender, ethnicity, and comorbidities contribute to heterogeneity by creating differences in how patients respond to treatments.
What is the impact of high levels of within-group heterogeneity on subgroup analyses?
High levels of within-group heterogeneity can limit the effectiveness of subgroup analyses, indicating substantial variation in study outcomes.
Can you provide an example of how heterogeneity affects clinical trial outcomes?
An example is a medication that shows significant effectiveness in one patient group but yields different outcomes in another group, highlighting the need for customized research strategies.
What did the systematic review and meta-analysis by Sabitova et al. reveal about heterogeneity?
The review revealed that geographical and demographic factors significantly influence study outcomes, with 32% of participants reporting high levels of job burnout, which varied by country.
How does understanding heterogeneity enhance healthcare research?
Comprehending heterogeneity enhances the relevance and applicability of research findings in healthcare by ensuring that treatments and strategies are tailored to diverse patient populations.