


In vivo testing is essential in clinical research, providing critical insights into the safety, efficacy, and biological interactions of new therapies within living organisms. This article outlines the structured phases of in vivo testing, from preclinical studies to post-marketing surveillance, emphasizing its role in anticipating human reactions and guiding drug development. It also addresses the challenges and methodologies involved in the process, highlighting the importance of collaboration in overcoming these obstacles. As we navigate the complexities of the Medtech landscape, understanding the intricacies of in vivo testing becomes paramount for advancing therapeutic innovations.
In the intricate landscape of clinical research, in vivo testing stands as a cornerstone, offering invaluable insights into the performance of treatments within living organisms. This guide delves into the multifaceted world of in vivo testing, illuminating its critical phases, methodologies, and the challenges researchers encounter in this essential process. As the demand for effective therapies escalates, it prompts a pivotal question: how can researchers navigate the complexities of in vivo testing to ensure safety and efficacy while adhering to ethical standards?
In a living system, an in vivo test refers to experiments performed within a living organism, such as animals or humans, to evaluate the impacts of a medication or treatment. This type of in vivo test is crucial in clinical research, as it provides insights into the pharmacokinetics, pharmacodynamics, and overall effectiveness of a substance in a biological context. In contrast to in vitro testing, which occurs in regulated settings outside of a living organism, in situ research provides a more accurate understanding of how a drug interacts with biological systems. The significance of in vivo tests lies in their capability to anticipate human reactions, identify potential side effects, and determine safe dosage levels, ultimately guiding the development of effective and safe therapeutic interventions.
Companies like bioaccess® play a pivotal role in this process by facilitating accelerated clinical trials for Medtech, Biopharma, and Radiopharma startups. bioaccess® offers comprehensive services, including regulatory approval, patient recruitment, and connecting startups with top-ranked clinical research sites. For instance, Avantec Vascular has selected bioaccess™ to assist with their first-in-human clinical trial of an innovative vascular device in Latin America. This collaboration exemplifies how strategic partnerships can enhance the efficiency and effectiveness of in-body testing.
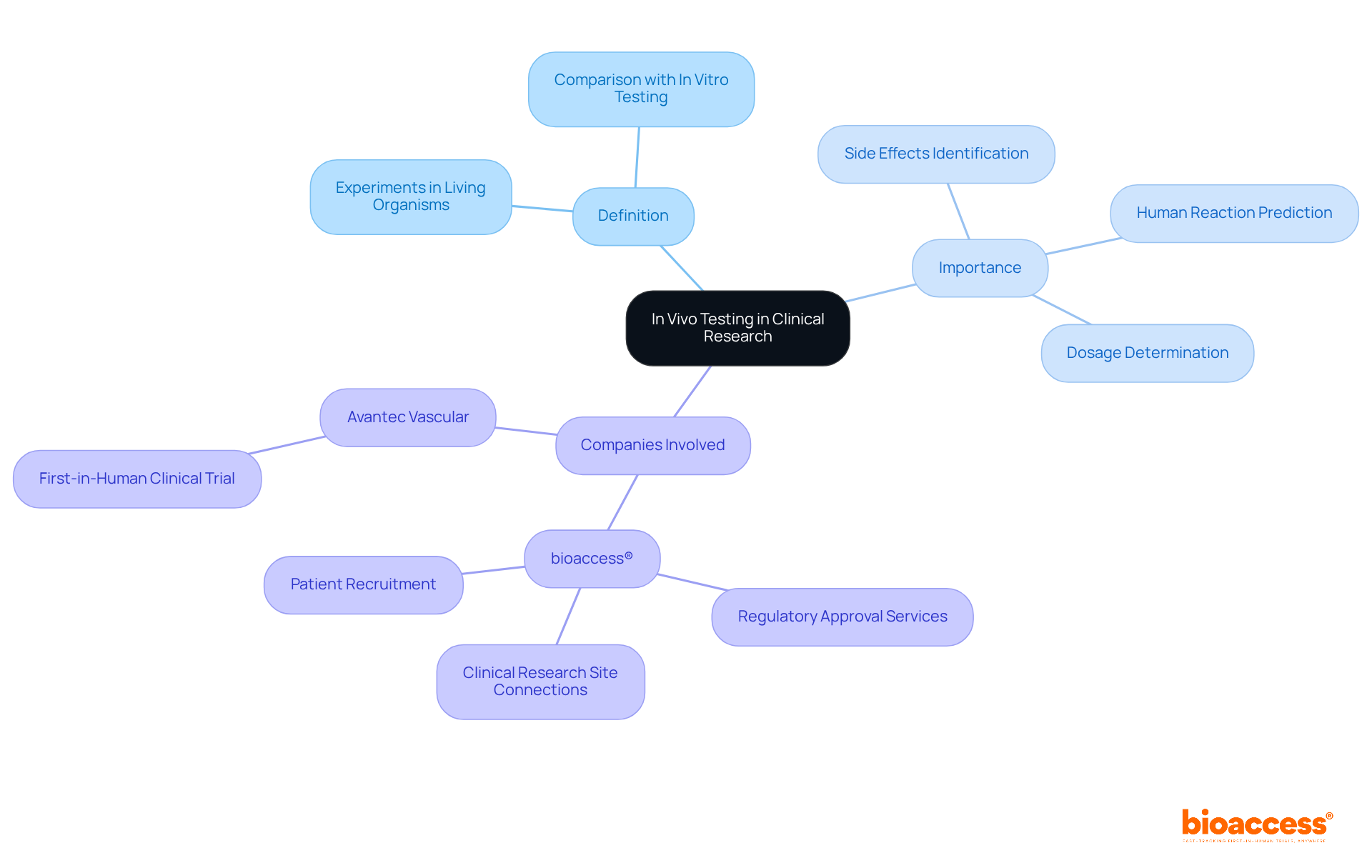
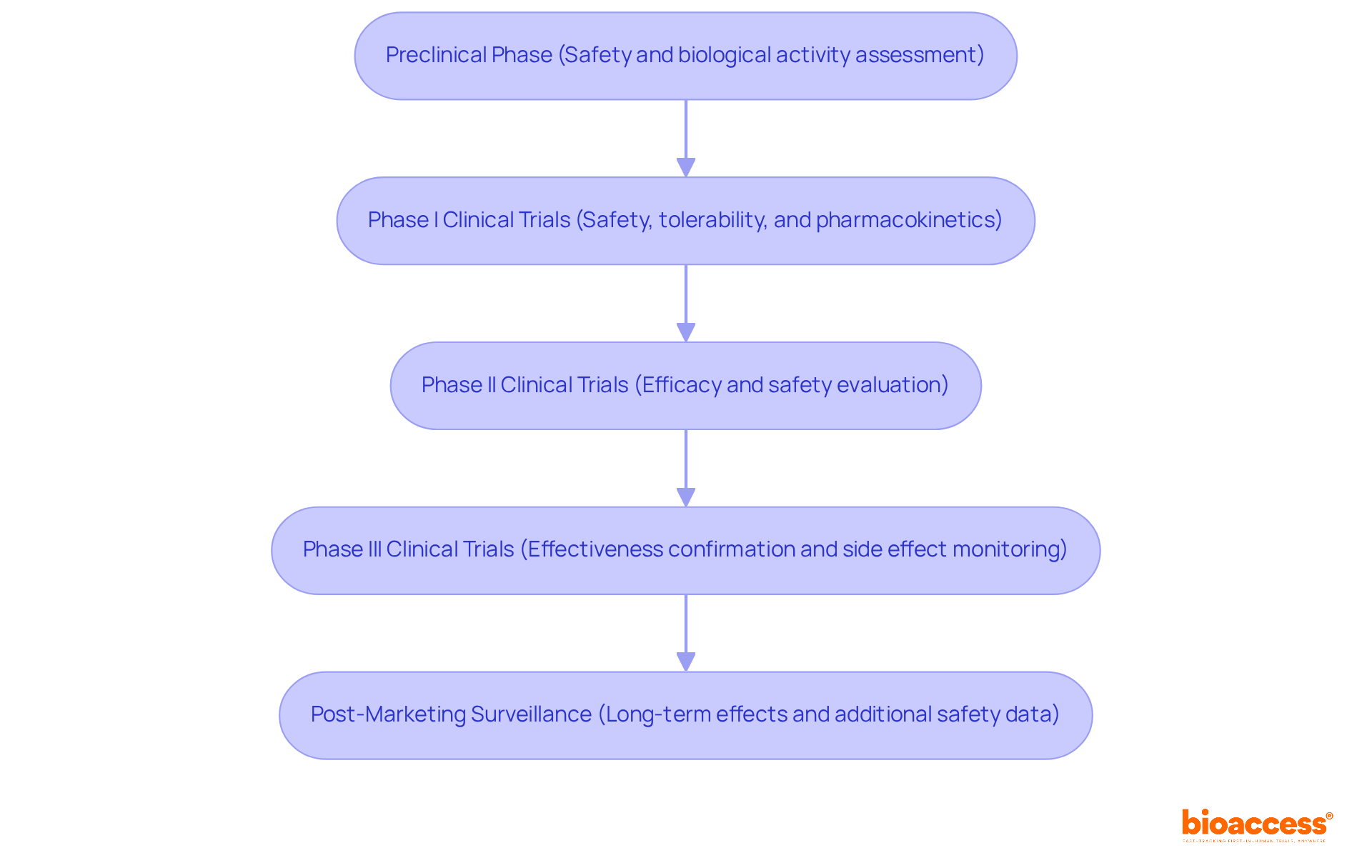
In vivo test in drug development typically involves several key phases that are critical for ensuring the safety and efficacy of new therapies.
The Preclinical Phase serves as the foundation of this process, encompassing laboratory and animal studies aimed at evaluating the safety and biological activity of candidate compounds. This phase meticulously assesses toxicity, pharmacokinetics, and pharmacodynamics, laying the groundwork for subsequent human trials.
Following this, Phase I Clinical Trials are conducted with a small group of healthy volunteers. This phase is pivotal as it focuses on safety, tolerability, and pharmacokinetics, determining the compound's effects on humans while identifying any potential adverse reactions.
In Phase II Clinical Trials, a larger cohort of patients with the targeted condition is involved. Here, the treatment's efficacy is evaluated alongside a further assessment of its safety, providing crucial insights into its therapeutic potential.
Phase III Clinical Trials expand the population size even further, aiming to confirm the treatment's effectiveness while monitoring side effects and comparing it to commonly used therapies. Successful completion of this phase is often a prerequisite for regulatory approval. Notably, with bioaccess®, clinical trials can achieve 50% faster patient enrollment, significantly addressing the recruitment challenges faced by medtech and biopharma startups.
Finally, Post-Marketing Surveillance plays an essential role after a drug's approval, with continuous in vivo tests conducted to observe long-term effects and gather additional safety and effectiveness data in the general population. Leveraging bioaccess®'s FDA-ready data can lead to $25K savings per patient, optimizing the overall clinical trial process and accelerating PMA data submissions by up to 11 months.
In clinical research, conducting in vivo tests is pivotal, involving several methodologies tailored to specific research objectives.
Animal Models: Selecting appropriate animal models is crucial. Typical models include rodents, rabbits, and non-human primates, chosen based on the disease under investigation and the mechanism of action.
Dosing Regimens: Establishing the correct dosing regimen is vital. This involves determining the route of administration (oral, intravenous, etc.), dosage, and frequency to closely mimic human exposure.
Monitoring and Data Collection: Continuous monitoring of subjects is essential to evaluate the substance's effects. This includes measuring vital signs, observing behavioral changes, and collecting biological samples for analysis.
Statistical Analysis: Employing robust statistical techniques to examine the data gathered during in vivo tests is crucial for deriving valid conclusions regarding the drug's safety and effectiveness.
Ethical Considerations: Adhering to ethical guidelines is paramount. This includes obtaining necessary approvals from ethics committees and ensuring humane treatment of animal subjects throughout the research process.

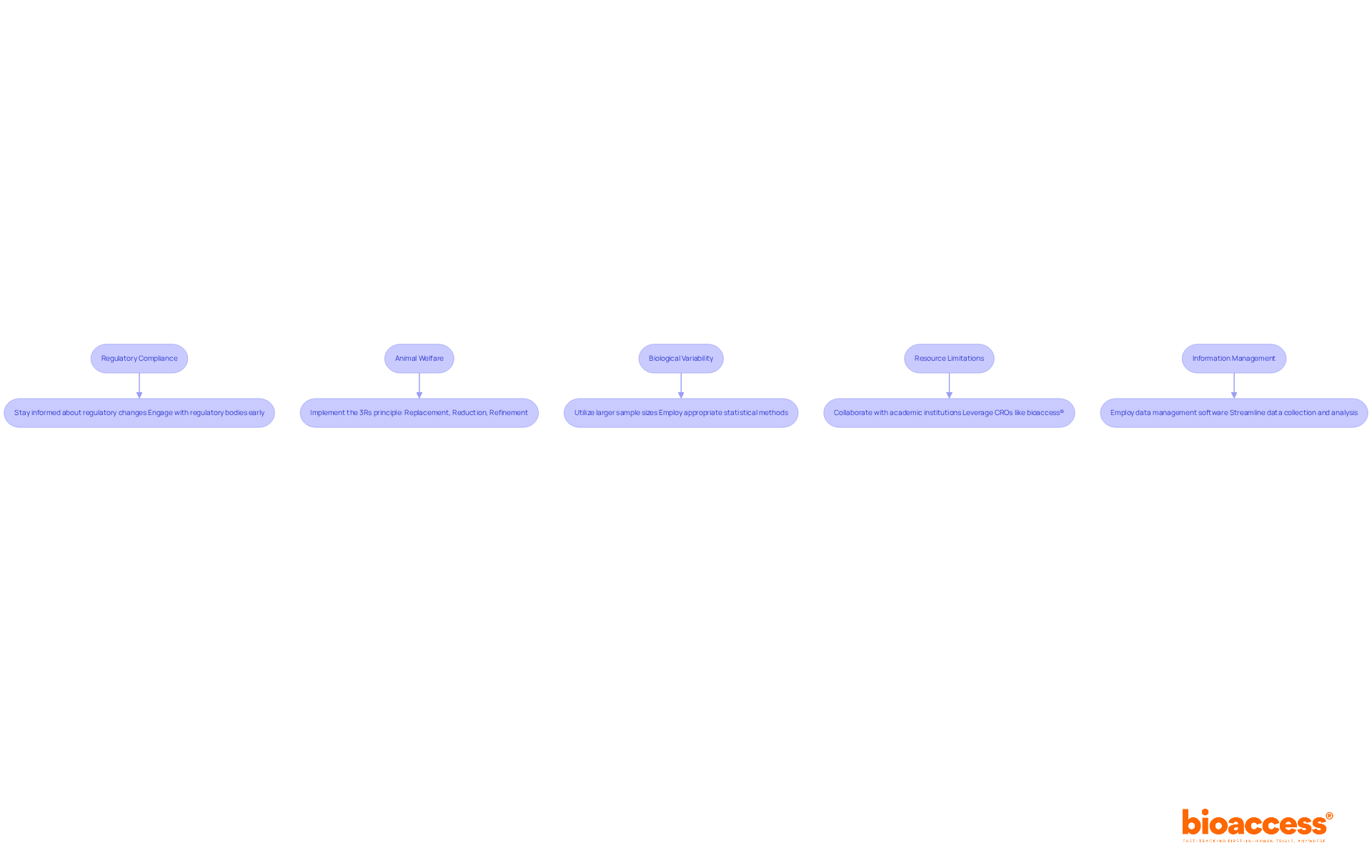
In vivo test challenges present several obstacles that researchers must navigate.
Regulatory compliance is a significant concern, as ensuring adherence to local and international regulations can be complex. To address this, it is essential to stay informed about regulatory changes and engage with regulatory bodies early in the design process.
Animal welfare concerns also play a crucial role in the ethics surrounding in vivo test research. Researchers must prioritize the ethical treatment of animal subjects during in vivo tests. Implementing the 3Rs principle—Replacement, Reduction, Refinement—can effectively minimize animal use while enhancing welfare standards.
Another challenge is the variability in biological responses among individual subjects, which can complicate data interpretation. Utilizing larger sample sizes and appropriate statistical methods is vital to account for this variability, ensuring robust conclusions.
Resource limitations, such as restricted access to facilities or funding, can hinder the execution of studies. Collaborating with academic institutions or contract research organizations (CROs) like bioaccess® allows researchers to leverage shared resources and expertise, overcoming these barriers.
Finally, information management poses a daunting task due to the extensive amounts of data involved. Employing data management software can streamline data collection, storage, and analysis, thereby ensuring accuracy and efficiency throughout the research process.
In vivo testing stands as a cornerstone in clinical research, providing invaluable insights into how treatments interact within living organisms. This comprehensive guide has illuminated the critical phases involved in drug development, from preclinical assessments to post-marketing surveillance, underscoring the necessity of in vivo tests for ensuring both safety and efficacy of new therapies.
Key arguments throughout this article have highlighted the structured methodology of in vivo testing, emphasizing the importance of selecting appropriate animal models, establishing effective dosing regimens, and adhering to ethical standards. Moreover, the challenges faced in this field—including regulatory compliance and animal welfare concerns—have been addressed alongside practical solutions that can enhance the overall efficiency and integrity of the testing process.
The significance of mastering in vivo testing cannot be overstated, as it shapes the future of therapeutic interventions and safeguards the health of patients. Researchers and organizations are encouraged to embrace strategic partnerships, such as those offered by bioaccess®, to streamline their clinical trials and overcome common obstacles. By prioritizing rigorous methodologies and ethical considerations, the path to developing safe and effective treatments can be significantly accelerated, ultimately benefiting the broader healthcare landscape.
What is in vivo testing?
In vivo testing refers to experiments conducted within a living organism, such as animals or humans, to evaluate the effects of a medication or treatment.
Why is in vivo testing important in clinical research?
In vivo testing is crucial in clinical research because it provides insights into pharmacokinetics, pharmacodynamics, and the overall effectiveness of a substance in a biological context, helping to predict human reactions, identify potential side effects, and determine safe dosage levels.
How does in vivo testing differ from in vitro testing?
In vivo testing occurs within a living organism, while in vitro testing takes place in controlled environments outside of a living organism. In vivo testing offers a more accurate understanding of how a drug interacts with biological systems.
What role does bioaccess® play in clinical trials?
bioaccess® facilitates accelerated clinical trials for Medtech, Biopharma, and Radiopharma startups by providing services such as regulatory approval, patient recruitment, and connecting startups with top-ranked clinical research sites.
Can you provide an example of a company using bioaccess® for in vivo testing?
Avantec Vascular has chosen bioaccess® to assist with their first-in-human clinical trial of an innovative vascular device in Latin America, demonstrating how strategic partnerships can enhance the efficiency of in-body testing.