


This article delineates the essential steps for clarifying and documenting indications for use in clinical research, a process that is critical for ensuring compliance and the successful deployment of medical devices. It outlines a structured approach that includes:
Ultimately, this meticulous process guarantees that devices meet both medical and ethical standards, reinforcing the importance of collaboration in navigating the complexities of the Medtech landscape.
Clarifying the indications for use in clinical research is not merely a regulatory necessity; it is a pivotal element that can significantly influence the success of medical devices and treatments. By mastering the process of defining these indications, researchers can enhance the credibility of their studies and ensure compliance with evolving regulatory standards.
However, the challenge lies in navigating the complexities of stakeholder engagement, ethical considerations, and documentation practices.
How can clinical researchers effectively balance these demands to optimize patient outcomes and streamline the approval process?
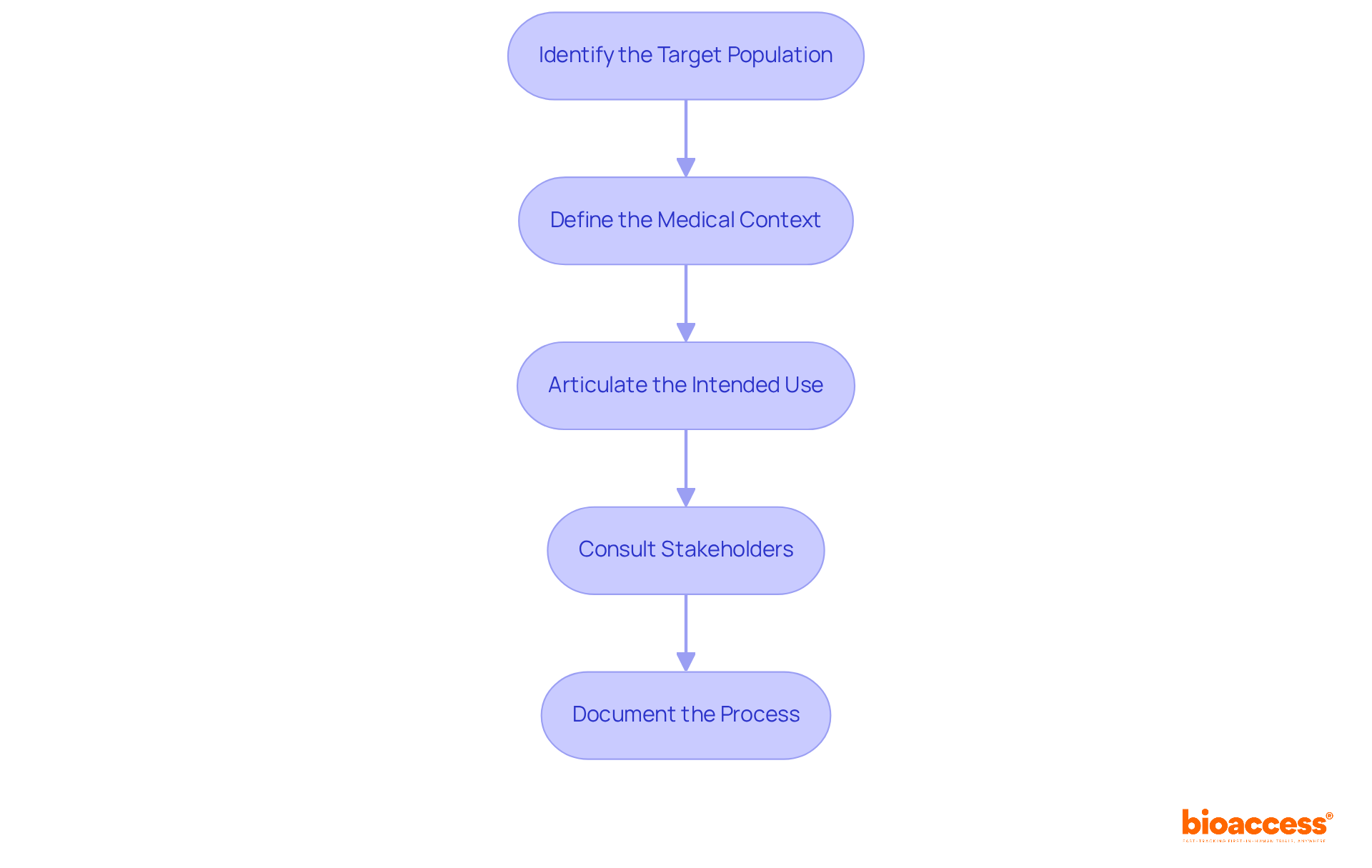
Indications for use outline the conditions or situations in which a medical device or treatment is intended to be utilized. To effectively clarify these indications, it is essential to follow these steps:
Identify the Target Population: Determine the demographic that will benefit from the device or treatment, considering factors such as age, gender, health status, and specific medical conditions. Successful examples underscore the importance of tailoring studies to diverse populations, ensuring broad applicability and effectiveness.
Define the Medical Context: Clearly outline the medical scenarios in which the device will be employed. A thorough understanding of the disease state and anticipated outcomes, along with the indications for use, significantly influences the device's acceptance and success in the market.
Articulate the Intended Use: Concisely state what the device or treatment is designed to achieve, focusing on its primary function. Clarity is essential, as the FDA increasingly requires accurate wording in statements of use to comply with the indications for use.
Consult Stakeholders: Engage with healthcare specialists, governing organizations, and potential users to validate the indications. Their insights are invaluable for enhancing the clarity and relevance of the IFU, ensuring it meets the indications for use as well as medical and compliance expectations.
Document the Process: Maintain a comprehensive record of discussions and decisions made during this clarification process. This documentation is crucial for ensuring transparency and adherence in later phases of research, facilitating smoother submissions to authorities.
By mastering these steps, medical researchers can enhance the credibility of their devices, streamline the approval process, and ultimately improve patient outcomes.
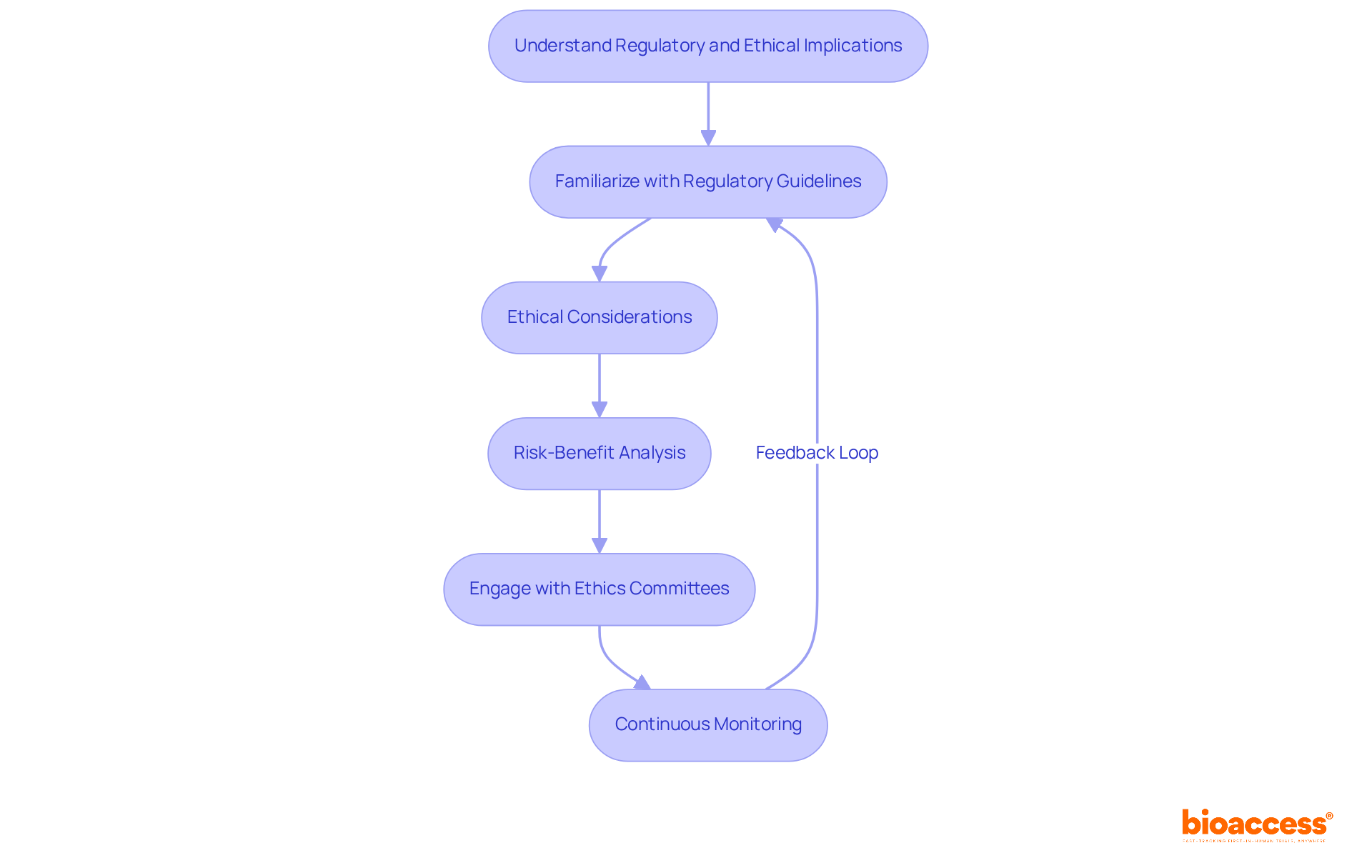
Understanding the regulatory and ethical consequences of usage is essential for ensuring compliance and safeguarding participant safety. To effectively navigate this complex landscape, consider the following strategies:
Familiarize with Regulatory Guidelines: Conduct a thorough review of regulations from authorities such as the FDA and EMA. These guidelines delineate the indications for use, which are crucial for ensuring adherence in clinical trials.
Ethical Considerations: Align practices with ethical standards, emphasizing informed consent and the minimization of risks to participants. The ethical implications are significant, especially as the FDA's evolving guidelines in 2025 underscore the necessity of robust survival data and patient-centric trial designs.
Risk-Benefit Analysis: Perform a comprehensive risk-benefit analysis to assess the potential advantages of the device or treatment against the associated risks. This analysis should be meticulously documented and revisited throughout the study to ensure ongoing relevance and accuracy.
Engage with Ethics Committees: Present the rationale for use to an ethics committee for thorough review. Their insights can shed light on ethical considerations that may not have been initially recognized, thereby enhancing the integrity of the trial.
Continuous Monitoring: Maintain vigilant oversight of compliance with regulatory and ethical standards throughout the research process. Be prepared to adjust the indications for use according to new findings or feedback, ensuring that participant safety remains the top priority.
Incorporating comprehensive trial management services—such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—can significantly enhance the effectiveness of these strategies. Adhering to regulatory guidelines improves compliance rates in research trials, highlighting the critical importance of these practices. By integrating these strategies, research professionals can adeptly navigate the ethical landscape, fostering trust and safety in their studies.
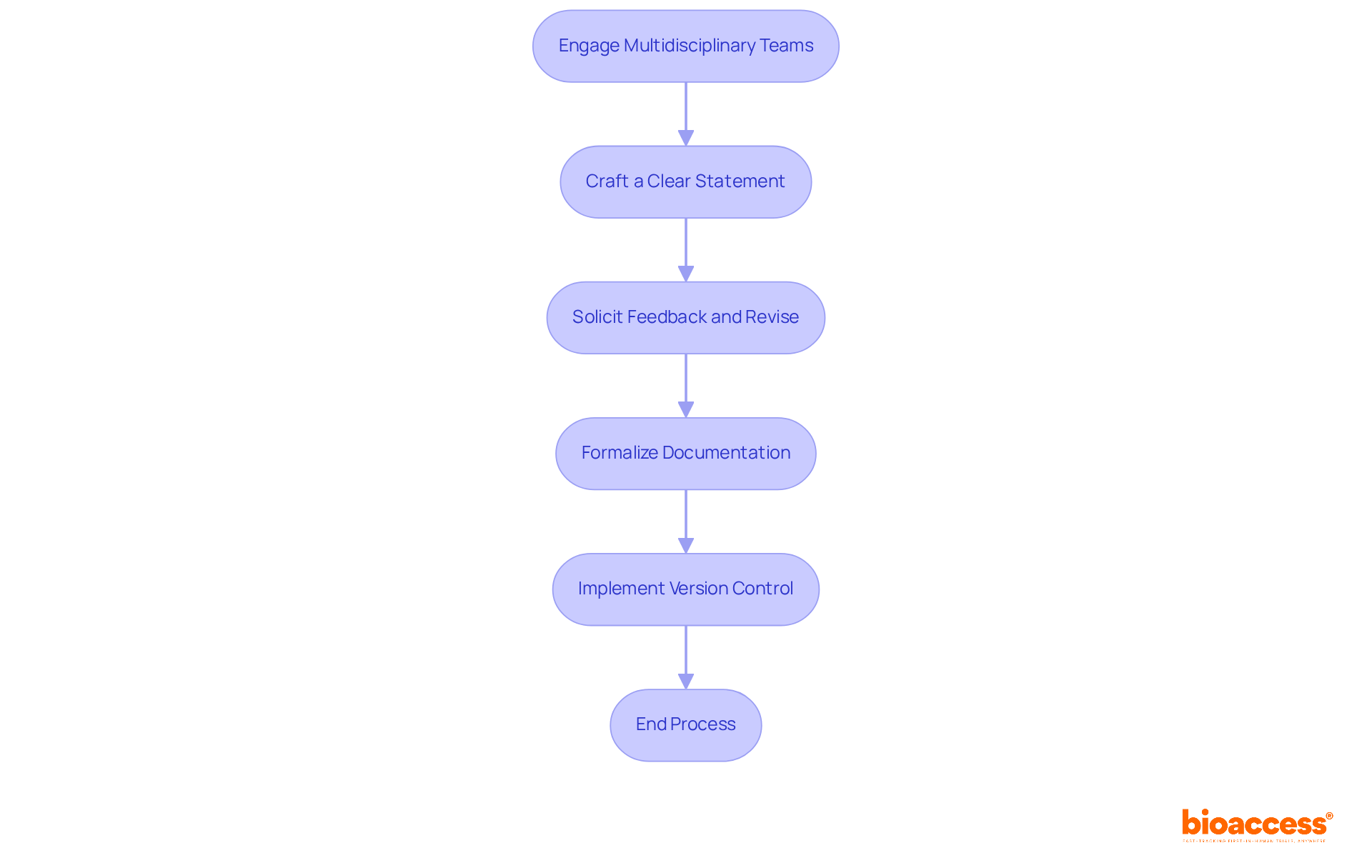
To effectively determine and document indications for use, organizations must adhere to the following best practices:
Engage Multidisciplinary Teams: Collaborating with clinical, compliance, and marketing professionals is essential to gather a broad spectrum of insights on the uses. This diverse input is crucial for a comprehensive understanding of the product's intended use, particularly within the context of INVIMA's regulatory framework, which oversees health product compliance in Colombia.
Craft a Clear Statement: It is imperative to develop a concise and straightforward statement that clearly expresses the purposes for use. Avoiding technical jargon ensures that the statement is accessible to all stakeholders, aligning with the clarity emphasized by INVIMA.
Solicit Feedback and Revise: Sharing the draft with relevant stakeholders for their feedback is vital. Incorporating their suggestions enhances the document's accuracy and comprehensiveness, ensuring it reflects a consensus view that meets the standards set by INVIMA's Directorate for Medical Devices and other Technologies.
Formalize Documentation: Once the statement is finalized, it should be recorded in a formal report. This report must outline the reasoning behind the signals, include supporting data, and reference relevant guidelines from INVIMA, acknowledged as a Level 4 health authority by PAHO/WHO.
Implement Version Control: Establishing a version control system to track modifications to the usage guidelines is crucial. This practice ensures that all stakeholders have access to the most current information and can reference it as needed.
By following these steps, organizations can ensure that their indications for use are accurately documented, well-defined, and compliant with expectations, ultimately enhancing the effectiveness of clinical trials. The importance of thorough documentation is underscored by statistics indicating that approximately 20% of drugs acquire new black box warnings post-marketing, highlighting the need for meticulous collaboration and adherence to regulatory standards. Furthermore, referencing authoritative sources like the ICH E9(R1) addendum, which emphasizes clarity in trial objectives, enhances the credibility of these best practices.
Mastering the indications for use in clinical research is essential for ensuring that medical devices and treatments are utilized effectively and safely. This guide outlines the critical steps involved in clarifying these indications, emphasizing the importance of a well-defined framework that aligns with regulatory requirements and ethical standards.
Key insights from the article highlight the necessity of:
Engaging stakeholders and documenting the entire process are vital for maintaining transparency and compliance. Furthermore, understanding regulatory and ethical implications plays a crucial role in safeguarding participant safety and ensuring the credibility of clinical trials.
In conclusion, the significance of accurately determining and documenting indications for use cannot be overstated. By implementing best practices and adhering to regulatory guidelines, researchers can enhance the effectiveness of their studies, foster trust among stakeholders, and ultimately contribute to improved patient outcomes. Embracing these strategies not only streamlines the approval process but also reinforces the commitment to ethical research practices, paving the way for advancements in healthcare.
What are indications for use in clinical research?
Indications for use outline the conditions or situations in which a medical device or treatment is intended to be utilized.
How can researchers clarify indications for use?
Researchers can clarify indications for use by identifying the target population, defining the medical context, articulating the intended use, consulting stakeholders, and documenting the process.
Why is it important to identify the target population?
Identifying the target population is crucial because it determines the demographic that will benefit from the device or treatment, ensuring the study is tailored to diverse populations for broad applicability and effectiveness.
What should be included when defining the medical context?
When defining the medical context, it is important to outline the medical scenarios in which the device will be employed, understand the disease state, and anticipate outcomes.
What does articulating the intended use involve?
Articulating the intended use involves concisely stating what the device or treatment is designed to achieve, with a focus on its primary function and ensuring clarity to comply with FDA requirements.
Why is consulting stakeholders necessary?
Consulting stakeholders, including healthcare specialists and governing organizations, is necessary to validate the indications and enhance the clarity and relevance of the instructions for use (IFU).
What is the significance of documenting the clarification process?
Documenting the clarification process is significant for maintaining a comprehensive record of discussions and decisions, which ensures transparency and adherence in later phases of research and facilitates smoother submissions to authorities.