


The article underscores the critical importance of understanding and adhering to INVIMA guidelines for medtech companies engaged in clinical trials in Colombia. It asserts that compliance with these guidelines is essential for avoiding severe penalties and successfully navigating the clinical trial approval process. This adherence ultimately leads to more efficient research and enhanced market access for innovative medical devices. By grasping these guidelines, companies position themselves to thrive in a competitive landscape, ensuring their contributions to healthcare innovation are both impactful and compliant.
In the dynamic landscape of medical technology, understanding regulatory frameworks is paramount for success, particularly in emerging markets like Colombia. The National Food and Drug Surveillance Institute (INVIMA) plays a critical role in shaping the clinical trial environment, ensuring that medical devices and pharmaceuticals meet rigorous safety and efficacy standards. As demand for clinical trials in Latin America surges, with projections indicating substantial foreign investment, the importance of INVIMA's guidelines cannot be overstated.
Navigating this complex regulatory terrain requires a comprehensive grasp of essential documentation, compliance obligations, and the approval process. By delving into INVIMA's requirements, Medtech companies can not only streamline their operations but also enhance their potential for successful outcomes, ultimately contributing to improved healthcare access and innovation in the region.
The National Food and Drug Surveillance Institute serves as Colombia's regulatory authority, ensuring the safety, efficacy, and quality of medical devices and pharmaceuticals. Its influence is vital in the research landscape, as it establishes detailed INVIMA guidelines for medtech companies that must be followed for conformity with both national and international standards. This process involves a comprehensive assessment of research protocols, ongoing oversight of current projects, and rigorous adherence to ethical research standards.
As we look to 2025, the organization's role is becoming increasingly significant, particularly as the demand for research in Latin America rises. Specialists anticipate that Mexico alone could attract as much as $800 million annually in overseas investments for research studies, underscoring the potential for similar growth in Colombia. By understanding the INVIMA guidelines for medtech companies, firms can skillfully navigate the complexities of clinical trials in Colombia, leading to more efficient processes and favorable outcomes. A key component of this framework is the Clinical Trial Application (CTA), which outlines the purpose of the research and includes all pertinent information, such as objectives and methodologies. Non-compliance with the INVIMA guidelines for medtech companies can result in severe penalties, including fines, product seizures, and even facility shutdowns, emphasizing the critical nature of adhering to these guidelines.
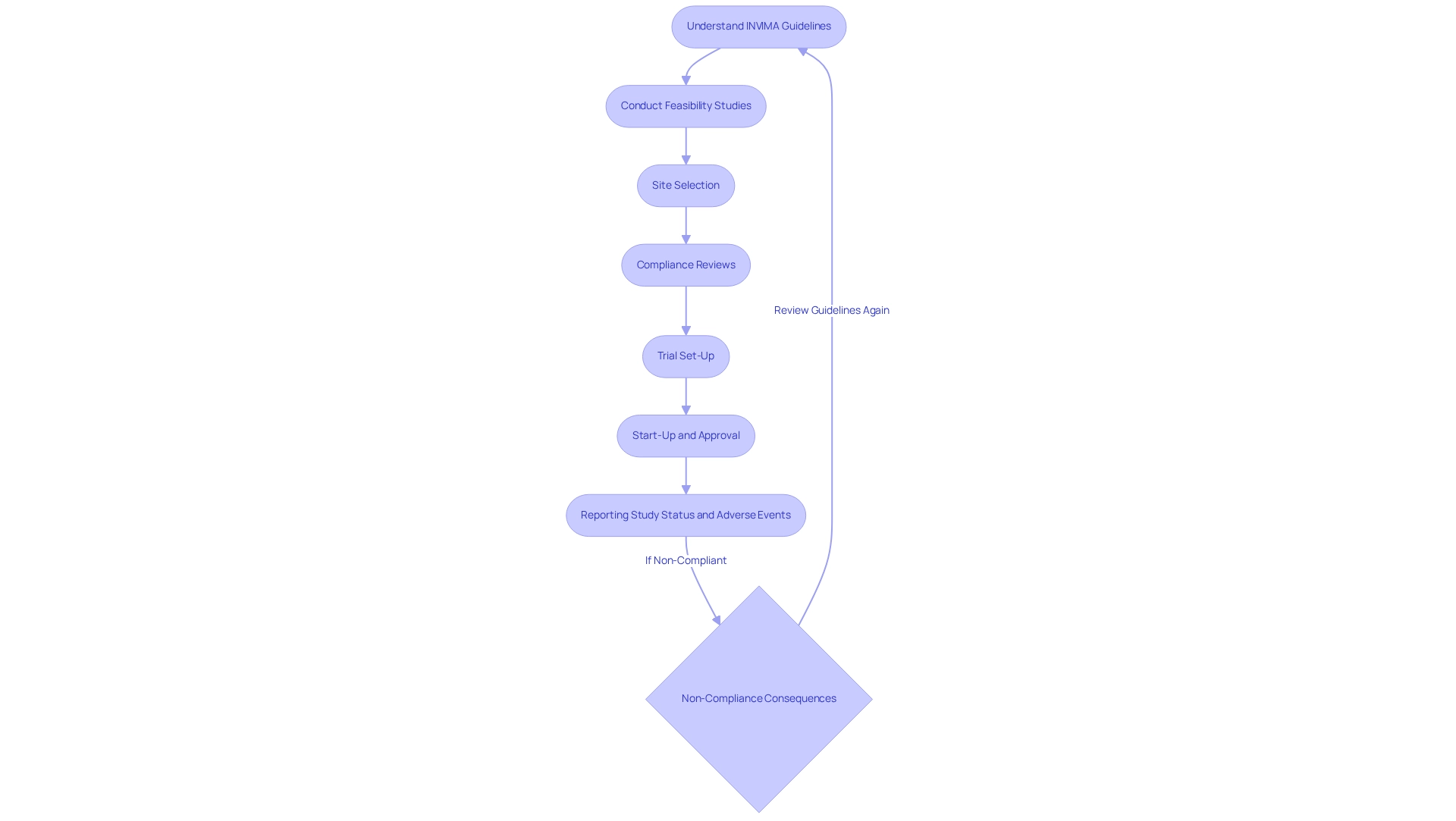
Successful Medtech companies demonstrate that a proactive approach to the INVIMA guidelines for medtech companies—including feasibility studies, site selection, compliance reviews, trial set-up, start-up, and approval, as well as reporting on study status and adverse events—not only mitigates risks but also accelerates the path to market for innovative medical devices. For example, understanding the financial implications, such as government fees for marketing authorization—ranging from $3,200 for drug products to $12,035 for GMP certificates—can further refine compliance strategies. Therefore, a thorough understanding of the INVIMA guidelines for medtech companies is essential for any Medtech firm aiming to conduct clinical research in Colombia. Reach out to us to discover how bioaccess can support you with regulatory compliance.
To comply with INVIMA regulations, Medtech companies must prepare and submit several essential documents, including:
These documents must be meticulously prepared to meet the INVIMA guidelines for medtech companies, ensuring a smooth approval process. A thorough registration file is essential, as it contains technical documentation, health data, and evidence of quality management systems, which align with the INVIMA guidelines for medtech companies, as emphasized in the case analysis on documentation prerequisites for approval. This thorough approach not only facilitates successful submissions but also promotes public health and safety by ensuring that all medical devices meet established safety and efficacy standards.
Statistics indicate that companies must import devices within three years of regulatory approval, or the health authority may cancel their registration. This underscores the importance of timely and accurate documentation, which is critical for survival and success in the Medtech sector. Additionally, bioaccess provides extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, which are vital for effectively navigating the regulatory environment. The introduction of the electronic adverse events reporting tool in August 2015 has streamlined the reporting process, making it more efficient and cost-effective for Medtech companies. As Katherine Ruiz, an expert in regulatory affairs, emphasizes, "A comprehensive approach to data management is essential for success in the evolving MedTech landscape." By adhering to these documentation requirements and leveraging bioaccess's expertise, companies can navigate the regulatory landscape effectively and advance their medical devices to market.

The clinical trial approval process in Colombia encompasses several essential steps that Medtech companies must navigate effectively.
Organizations like bioaccess® play a pivotal role in offering management services for research in Colombia, including feasibility assessments, project management, and compliance reviews, providing essential support for Medtech companies as they navigate this complex approval landscape. Furthermore, the partnership between bioaccess and Caribbean Health Group aims to establish Barranquilla as a premier location for medical studies, backed by the Minister of Health's initiative to enhance research in the area. As Ms. Markham, CEO of Clinlogix, emphasizes, navigating the approval process is vital for ensuring that innovative solutions reach those in need. Addressing the inequalities in healthcare access highlighted in the case study 'Challenges in Healthcare Access' underscores the significance of the approval process for medical studies in improving healthcare outcomes.
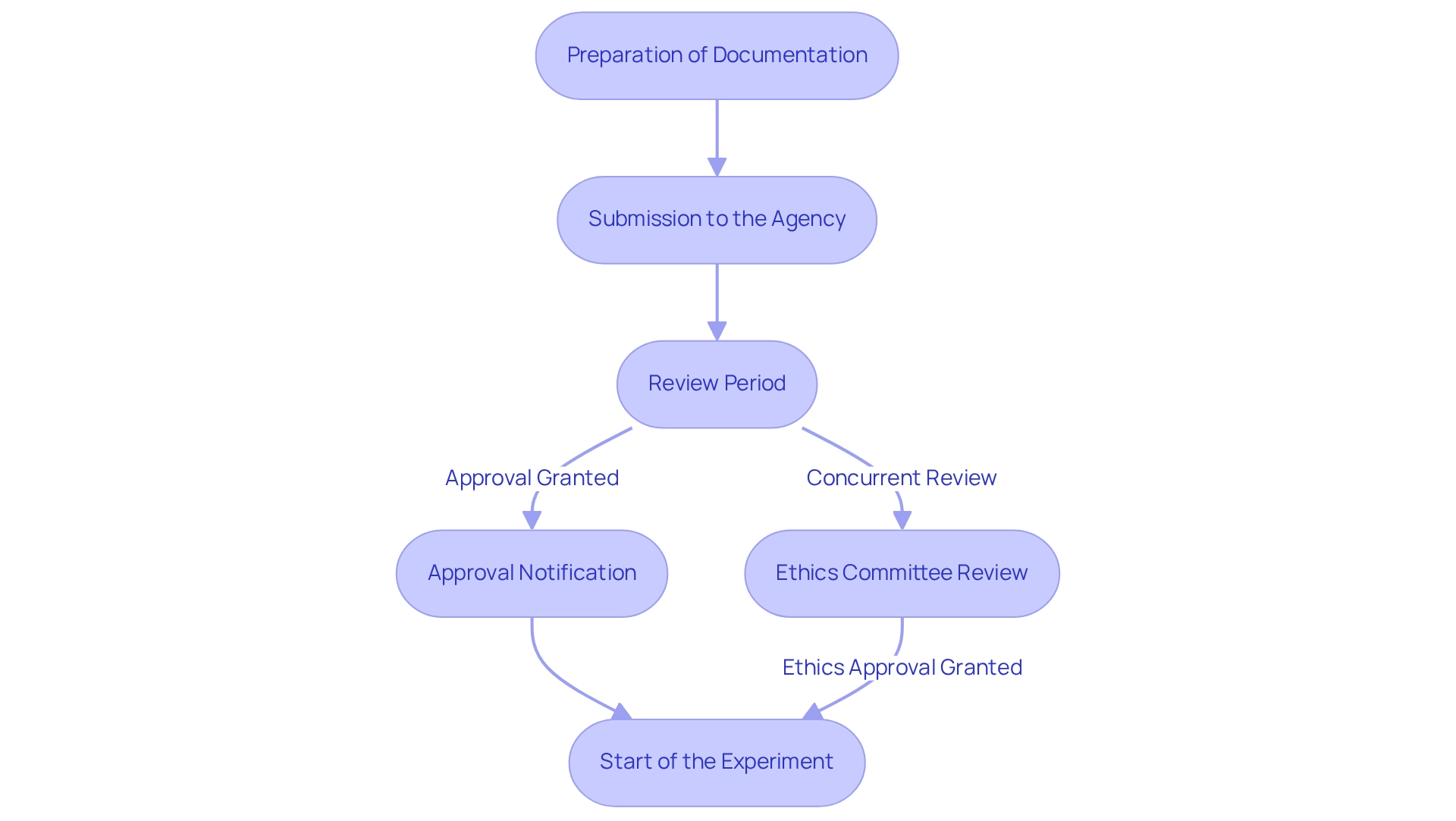
Following the receipt of authorization from the regulatory body, Medtech firms are obligated to fulfill several critical post-approval compliance and reporting responsibilities to ensure the integrity of clinical studies and the safety of participants.
Ongoing Monitoring: It is mandatory to conduct continuous assessments of the medical device's safety and efficacy throughout the trial period.
Adverse Event Reporting: Serious adverse events must be reported to INVIMA within 72 hours of awareness, while non-serious events should be communicated within eight days. This timely reporting is essential for safeguarding participant safety and ensuring regulatory compliance. A case analysis aimed at enhancing the quality of adverse event (AE) reports underscores the importance of including comprehensive information, such as medication details and the timing of events, to facilitate the evaluation of adverse events and improve patient safety.
Yearly Reports: Companies are required to submit annual reports that detail the progress of the clinical research, including participant safety metrics and any protocol changes. This transparency is crucial for regulatory oversight and fosters stakeholder confidence. Notably, Colombia provides a 100% tax deduction for investments in science, technology, and innovation projects, presenting a compelling incentive for Medtech companies to engage in diligent post-approval compliance.
Final Study Report: Upon conclusion of the trial, a comprehensive final report summarizing the study's findings and outcomes must be submitted to the regulatory authority. This report is vital for assessing the overall success and safety of the medical device. Adhering to these obligations not only guarantees compliance with the INVIMA guidelines for medtech companies but also cultivates trust with stakeholders and participants. As Colombia positions itself as a leading center for medical research, this commitment to transparency and safety is paramount in advancing medical knowledge and fostering innovation within the Medtech sector. The collaboration between bioaccess and Caribbean Health Group aims to establish Barranquilla as a key location for medical studies in Latin America, bolstered by the support of the nation's Minister of Health, further enhancing the region's appeal for clinical trials.

The landscape of clinical trials in Colombia is profoundly influenced by the regulatory frameworks established by the National Food and Drug Surveillance Institute (INVIMA). Understanding INVIMA's comprehensive guidelines is essential for Medtech companies striving to navigate the complexities of clinical trials effectively. The necessity for meticulous documentation, strict adherence to compliance obligations, and a thorough understanding of the approval process is paramount, as these elements are crucial for ensuring the safety and efficacy of medical devices and pharmaceuticals.
Furthermore, the post-approval compliance and reporting obligations highlight the critical importance of ongoing vigilance and transparency in clinical research. By fulfilling these responsibilities, companies not only comply with regulatory standards but also enhance the overall integrity of the healthcare system. The proactive strategies adopted by successful Medtech firms demonstrate that thorough preparation and strategic navigation of INVIMA’s requirements can significantly improve operational efficiency and expedite market entry.
As Colombia continues to rise as a pivotal player in the Latin American clinical trial landscape, the significance of INVIMA will only increase. By aligning with INVIMA's standards and leveraging the expertise of organizations like bioaccess, Medtech companies can ensure compliance while fostering innovation and enhancing healthcare access throughout the region. Embracing these practices is essential for driving advancements in medical technology, ultimately benefiting patients and the healthcare system at large.
What is the role of the National Food and Drug Surveillance Institute (INVIMA) in Colombia?
INVIMA serves as Colombia's regulatory authority, ensuring the safety, efficacy, and quality of medical devices and pharmaceuticals. It establishes detailed guidelines that medtech companies must follow to comply with national and international standards.
What are the key components of the INVIMA guidelines for medtech companies?
The INVIMA guidelines include a comprehensive assessment of research protocols, ongoing oversight of current projects, and adherence to ethical research standards. A key component is the Clinical Trial Application (CTA), which outlines the research purpose and includes objectives and methodologies.
What are the consequences of non-compliance with INVIMA guidelines?
Non-compliance with INVIMA guidelines can result in severe penalties, including fines, product seizures, and facility shutdowns.
How can understanding INVIMA guidelines benefit medtech companies conducting clinical trials in Colombia?
Understanding INVIMA guidelines helps medtech companies navigate the complexities of clinical trials, leading to more efficient processes and favorable outcomes. It enables proactive measures such as feasibility studies and compliance reviews, which can accelerate the path to market for innovative medical devices.
What financial implications should medtech companies be aware of regarding INVIMA compliance?
Medtech companies should consider government fees for marketing authorization, which range from $3,200 for drug products to $12,035 for GMP certificates, as these can impact compliance strategies.
Why is the role of INVIMA expected to become more significant by 2025?
The demand for research in Latin America is rising, with specialists estimating that Mexico could attract up to $800 million annually in overseas investments for research studies, indicating potential growth for similar investments in Colombia.