


Mastering ISF clinical trial management is crucial for success in clinical research. It involves implementing key strategies such as:
These strategies not only enhance communication but also ensure adherence to regulatory standards.
By improving document management and participant outcomes, they ultimately lead to more successful clinical trials. In the ever-evolving Medtech landscape, understanding these elements is essential for addressing key challenges. Collaboration among stakeholders is vital, as it fosters an environment where these strategies can thrive.
As we navigate the complexities of clinical trials, it’s important to consider how these practices can be integrated into your operations. Are you ready to elevate your clinical trial management? Embracing these strategies will not only streamline processes but also enhance the overall success of your trials.
Effective management of the Investigator Site File (ISF) stands as a cornerstone of successful clinical trials. It ensures that essential documents are not only organized but also compliant with regulatory standards. By mastering ISF management, research teams can enhance communication, streamline audits, and ultimately improve patient outcomes through ethical and efficient study execution.
As the landscape of clinical research evolves, organizations must confront the pressing challenges of compliance and quality control in their ISF practices. How can they ensure trial integrity and success in this dynamic environment?
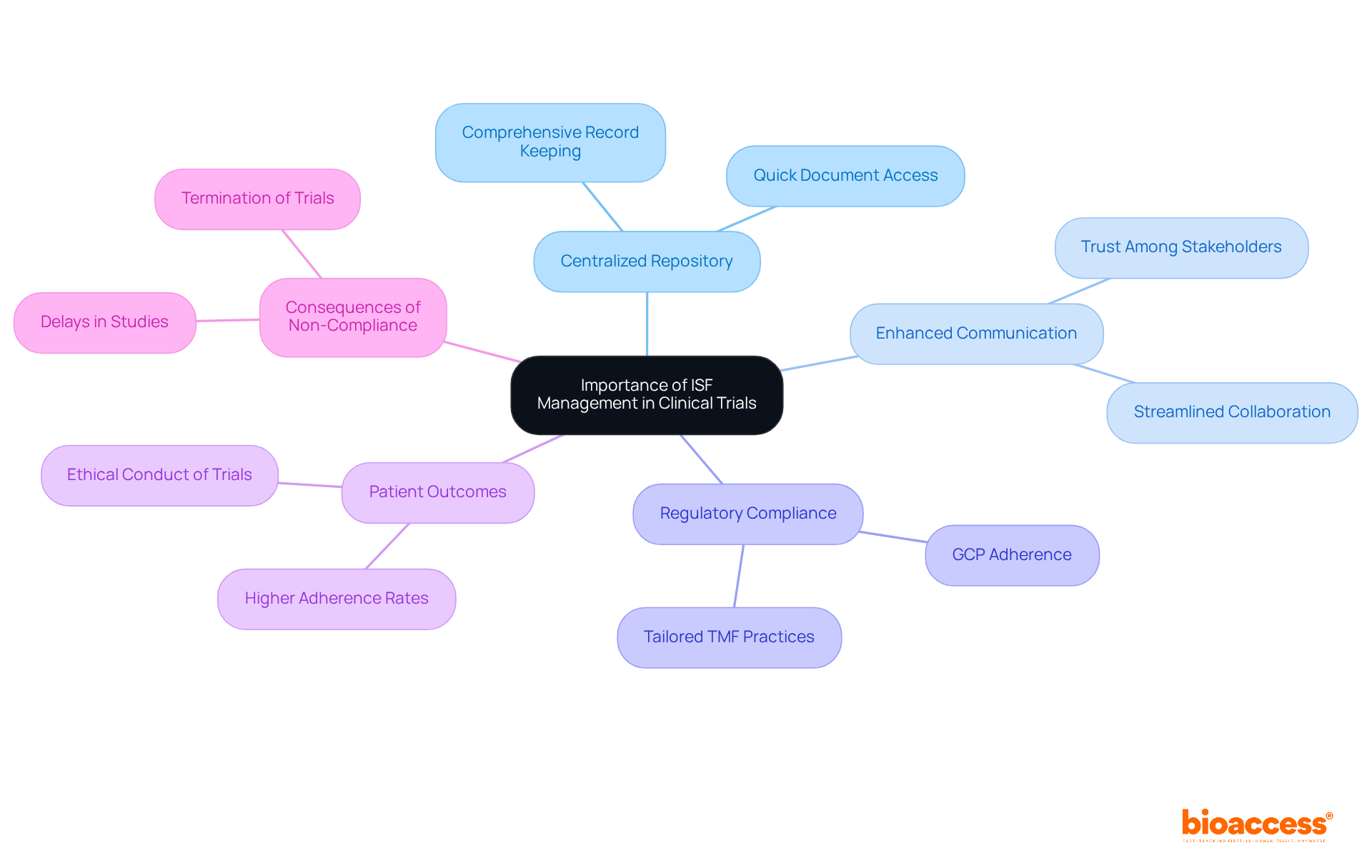
ISF clinical trial oversight is vital for the success of clinical studies, serving as a centralized repository for essential documents that uphold regulatory requirements and study protocols. Efficient management of the ISF clinical trial not only enhances communication between researchers and sponsors but also simplifies audits and fortifies the overall integrity of the study. By maintaining a well-organized ISF clinical trial, research teams can quickly access necessary documents, respond promptly to inquiries from regulatory bodies, and ensure precise documentation of all study activities. This proactive approach boosts the credibility of the research and fosters trust among stakeholders, including patients, sponsors, and regulatory authorities.
Moreover, a solid understanding of ISF oversight can significantly improve patient outcomes by ensuring that studies are conducted ethically and effectively. Real-world examples demonstrate that meticulous oversight in an ISF clinical trial leads to higher adherence rates during audits, ultimately strengthening the integrity and success of clinical studies. Familiarity with the specific TMF requirements of the overseeing regulatory agency is essential for sponsors and investigators, as it guarantees that the ISF aligns with regulatory expectations. Additionally, the ISF offers a localized perspective on trial execution, contributing to trial integrity.
Statistics show that adherence to Good Clinical Practice (GCP) guidelines is crucial for compliance, and effective ISF oversight plays a pivotal role in achieving this. As Negar Gharavi, Senior Director of Medical Writing & Regulatory Affairs, notes, "Organizations often adapt their TMF practices to align with regional regulations and guidance, ensuring that their TMF meets the expectations of the regulatory authorities." This highlights the importance of tailoring ISF management practices to meet specific regulatory requirements.
Furthermore, the potential risks associated with non-compliance in the ISF clinical trial oversight can lead to serious consequences, including delays and even termination of studies. By addressing common challenges in the ISF clinical trial oversight, clinical research teams can mitigate misuse of practices and enhance the overall success of clinical studies. At Bioaccess, our service offerings include:
All of which are crucial for effective ISF oversight.
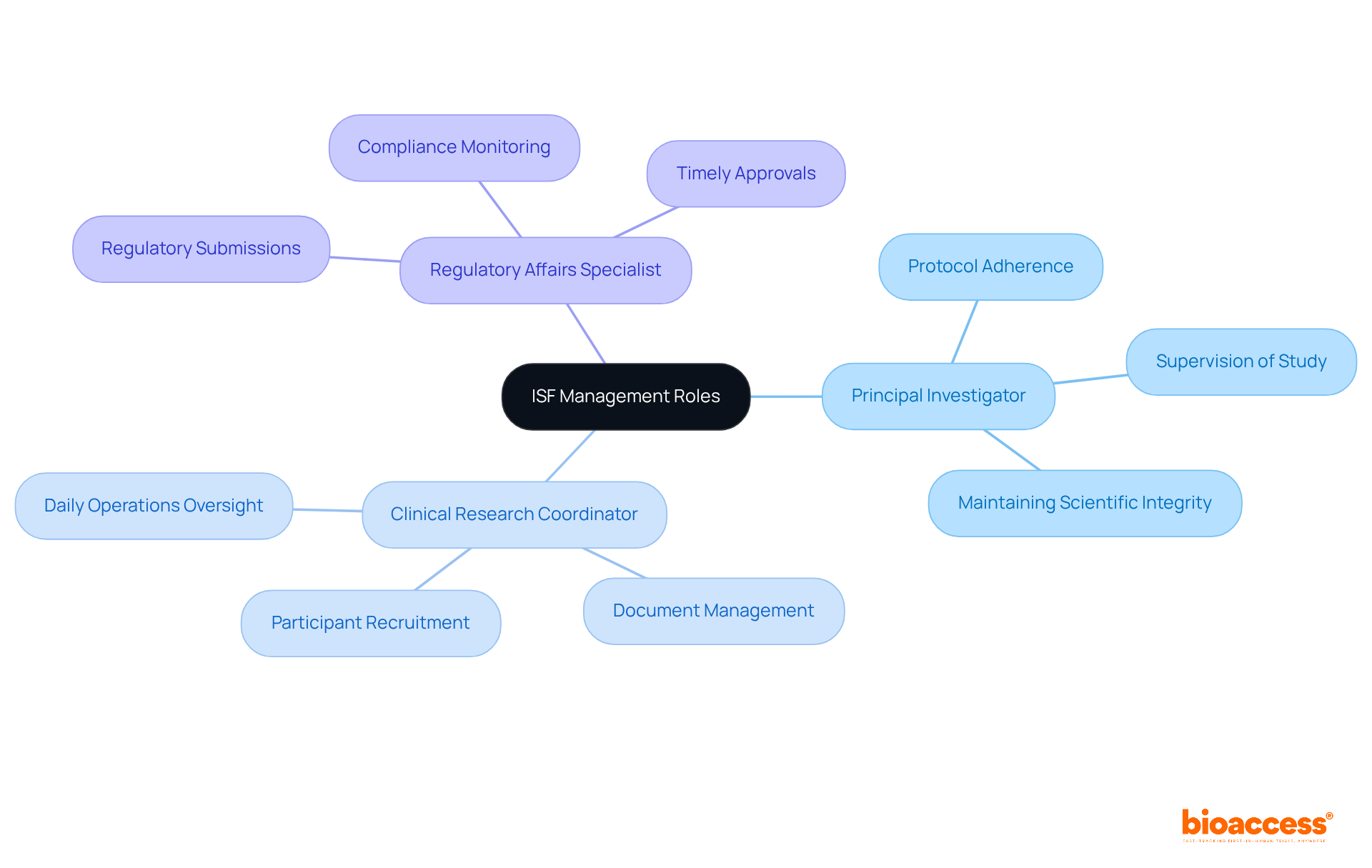
Effective management of the ISF clinical trial is crucial in clinical research, hinging on the establishment of clear roles and responsibilities. Each team member must understand their specific contributions to the study's success. The Principal Investigator (PI) supervises the study, ensuring adherence to protocols and maintaining scientific integrity. Meanwhile, the Clinical Research Coordinator (CRC) oversees daily operations, including participant recruitment and document management, while the Regulatory Affairs Specialist ensures timely and accurate regulatory submissions. By clearly defining these roles, teams can significantly enhance communication and collaboration, minimizing the risk of errors and omissions.
Regularly scheduled meetings are essential for reviewing responsibilities and addressing challenges, fostering accountability and teamwork. This organized approach not only enhances operational efficiency but also improves the quality of assessments, ensuring adherence to regulatory and ethical standards. For instance, organizations that have implemented defined roles report improved participant retention rates and streamlined processes, which are critical for the success of ISF clinical trials. Furthermore, utilizing Bioaccess's extensive clinical study oversight services—such as review and feedback on research documents, feasibility assessments, site selection, import permits, and project coordination—can further aid in the efficient execution of these roles. Ultimately, the influence of well-defined roles in ISF coordination is profound, leading to faster patient enrollment and more successful trial outcomes.
To achieve adherence and ensure quality control in Investigator Site File (ISF) management, organizations must adopt a systematic approach that includes:
Periodic audits of the ISF are crucial for identifying discrepancies and areas needing improvement, allowing teams to proactively tackle potential issues. Ongoing training for all team members on regulatory requirements and best practices is vital for upholding high standards of adherence. Furthermore, utilizing checklists can streamline the documentation process, ensuring that all necessary documents are present in the ISF and comply with regulatory standards. By fostering a culture focused on quality and compliance, organizations can significantly reduce the risk of non-compliance and enhance the overall credibility of their clinical trials.

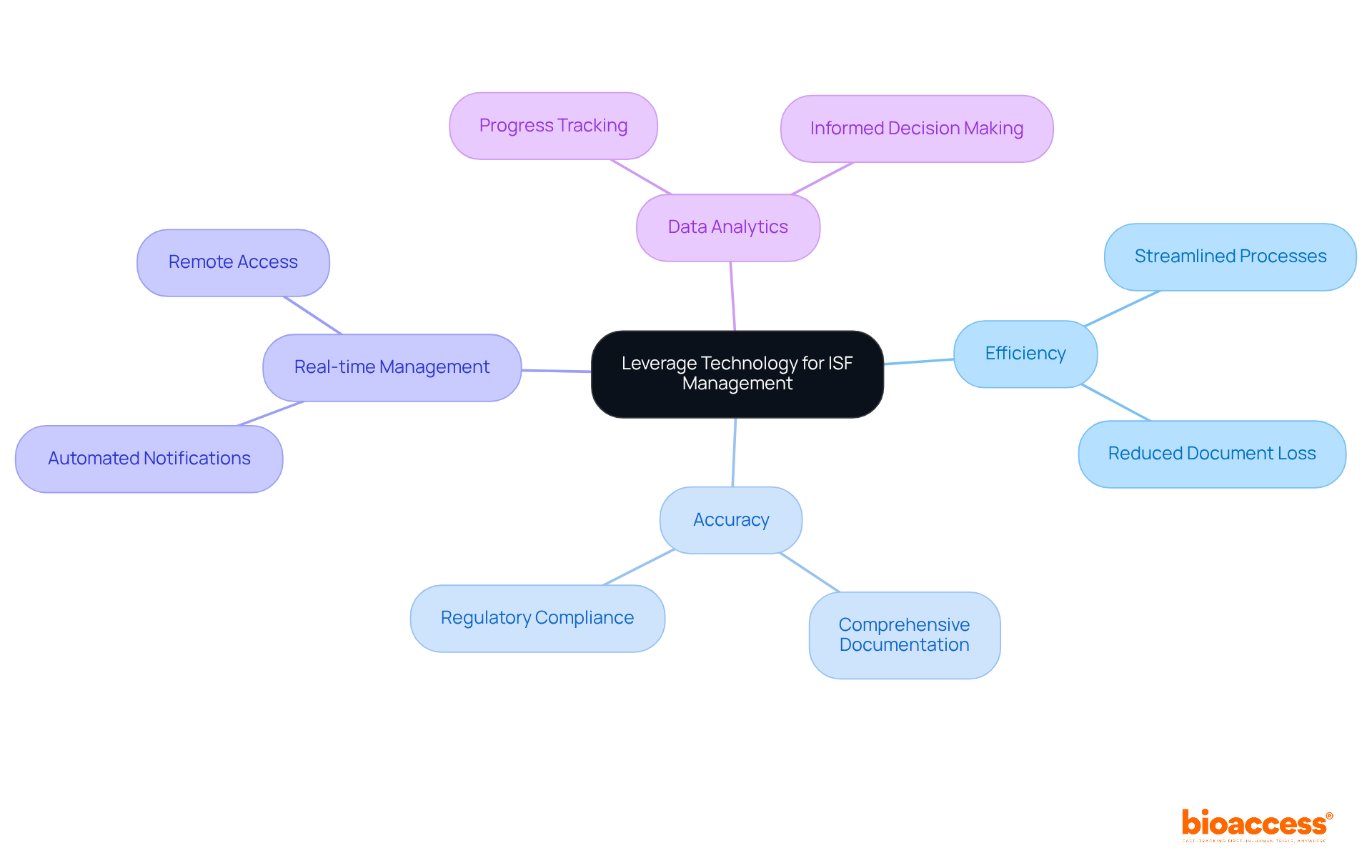
Incorporating technology into ISF operations significantly enhances efficiency and accuracy. Electronic ISF (eISF) systems facilitate real-time document management, allowing teams to access and update files from virtually anywhere. This capability reduces the risk of lost or outdated documents, a common challenge in ISF clinical trial research. Additionally, these systems often include features like automated notifications for document updates and regulatory checks, ensuring that all required materials remain current and comprehensive.
Moreover, leveraging data analytics tools provides valuable insights into progress and metrics, empowering teams to make informed decisions swiftly. By embracing technology, clinical research organizations such as bioaccess® can refine their processes for the ISF clinical trial. Their expertise in comprehensive clinical study management services—including:
positions them as leaders in the field. This strategic adoption ultimately leads to more successful trial outcomes and enhanced patient safety.
Mastering ISF clinical trial management is crucial for the success of clinical studies. A well-organized Investigator Site File (ISF) not only ensures compliance with regulatory standards but also enhances communication among stakeholders. This ultimately fosters trust and improves patient outcomes. The meticulous oversight of ISF management serves as a foundation for maintaining the integrity of clinical trials, positioning research teams to respond effectively to audits and regulatory inquiries.
The article highlights several key strategies for effective ISF management. These include:
Each of these elements plays a critical role in enhancing operational efficiency and ensuring that clinical trials adhere to Good Clinical Practice (GCP) guidelines. By adopting a systematic approach that incorporates regular audits, ongoing training, and advanced electronic systems, research organizations can mitigate risks associated with non-compliance and improve the overall credibility of their studies.
Reflecting on these insights, it becomes evident that effective ISF management is not merely a regulatory requirement; it is a strategic advantage that can significantly impact clinical trial outcomes. Organizations are encouraged to prioritize these best practices and embrace innovative technologies to enhance their ISF management processes. By doing so, they can improve operational efficiency and contribute to the advancement of clinical research, ultimately benefiting patients and the broader healthcare community.
What is the importance of ISF management in clinical trials?
ISF management is vital for the success of clinical studies as it serves as a centralized repository for essential documents that uphold regulatory requirements and study protocols. It enhances communication between researchers and sponsors, simplifies audits, and fortifies the overall integrity of the study.
How does efficient ISF management benefit research teams?
Efficient ISF management allows research teams to quickly access necessary documents, respond promptly to inquiries from regulatory bodies, and ensure precise documentation of all study activities, thereby boosting the credibility of the research and fostering trust among stakeholders.
What is the impact of ISF oversight on patient outcomes?
A solid understanding of ISF oversight can significantly improve patient outcomes by ensuring that studies are conducted ethically and effectively, leading to higher adherence rates during audits and strengthening the integrity and success of clinical studies.
Why is familiarity with TMF requirements important for sponsors and investigators?
Familiarity with the specific TMF requirements of the overseeing regulatory agency is essential as it guarantees that the ISF aligns with regulatory expectations, which is crucial for compliance with Good Clinical Practice (GCP) guidelines.
What are the risks associated with non-compliance in ISF clinical trial oversight?
Non-compliance in ISF clinical trial oversight can lead to serious consequences, including delays and even termination of studies.
What services does Bioaccess offer for effective ISF oversight?
Bioaccess offers services including feasibility studies, site selection, compliance reviews, study setup, import permits, project oversight, and comprehensive reporting, all of which are crucial for effective ISF oversight.