Overview
The article primarily addresses the critical steps required to ensure compliance with ISO 10993-7, which is essential for biocompatibility in medical devices. It presents a structured methodology that begins with:
- A thorough understanding of the standard.
- A detailed assessment of biocompatibility requirements.
- The necessity of comprehensive testing.
Additionally, it highlights the vital role of:
- Documentation
- Expert consultation
in effectively meeting regulatory standards.
Introduction
Navigating the complex landscape of medical device regulations can be daunting, particularly in ensuring biocompatibility. ISO 10993-7 is a critical standard within the ISO 10993 series, providing essential guidelines for evaluating the safety of materials used in medical instruments. This article presents a comprehensive roadmap for mastering ISO 10993-7, detailing the necessary steps to assess biocompatibility and comply with regulatory standards.
Manufacturers must not only meet these stringent requirements but also find ways to thrive in a competitive market.
Understand ISO 10993-7 Fundamentals
ISO 10993 7 is a crucial component of the ISO 10993 series, which is dedicated to the biological assessment of medical instruments. This standard specifically outlines the requirements for evaluating the suitability of materials used in medical devices. To master this standard, consider the following steps:
- Familiarize yourself with the standard by obtaining a copy of ISO 10993 7 and thoroughly reading the document to grasp its scope, definitions, and key concepts.
- Identify Key Terms: Pay close attention to terms such as 'biocompatibility', 'cytotoxicity', and 'material characterization'. Mastery of these terms is essential for effective communication and implementation.
- Review the Biological Evaluation Process: Comprehend the steps involved in the biological evaluation process, including risk assessment, testing, and documentation requirements.
- Study Relevant Guidelines: Familiarize yourself with related guidelines and standards, such as ISO 14971 (risk management) and ISO 10993-1 (general principles), as they provide critical context and additional requirements for compatibility assessments.
- Engage with industry resources by utilizing webinars, workshops, and industry publications to deepen your understanding of ISO 10993 7 and its real-world applications.

Assess Biocompatibility Requirements
To assess the biocompatibility requirements for your medical device, it is essential to follow these steps:
- Identify Classification: Begin by determining the classification of your medical equipment according to the regulatory guidelines established by INVIMA, the Colombia National Food and Drug Surveillance Institute. This classification significantly influences the compatibility assessment requirements.
- Evaluate Material Composition: Next, analyze the components utilized in your apparatus. Different materials may necessitate distinct biocompatibility tests, depending on their interaction with biological systems.
- Conduct a Risk Assessment: Perform a thorough risk evaluation to identify potential biological hazards associated with the equipment. Consider critical factors such as the duration of contact with tissue, the type of tissue involved, and the device's intended use.
- Select Appropriate Tests: Based on the findings from your risk evaluation, choose the suitable tests for compatibility as outlined in ISO 10993-7. Common tests include cytotoxicity, sensitization, and irritation assessments.
- Document Your Findings: It is crucial to maintain comprehensive documentation of your assessment process, including the rationale for selected tests and any results obtained. This documentation is vital for regulatory submissions and audits, particularly in compliance with INVIMA's standards.
- Consult with Specialists: If necessary, seek guidance from compatibility experts or regulatory advisors, such as Ana Criado, who possesses extensive experience in regulatory affairs and biomedical engineering. This will ensure that your evaluation meets all essential criteria and standards.

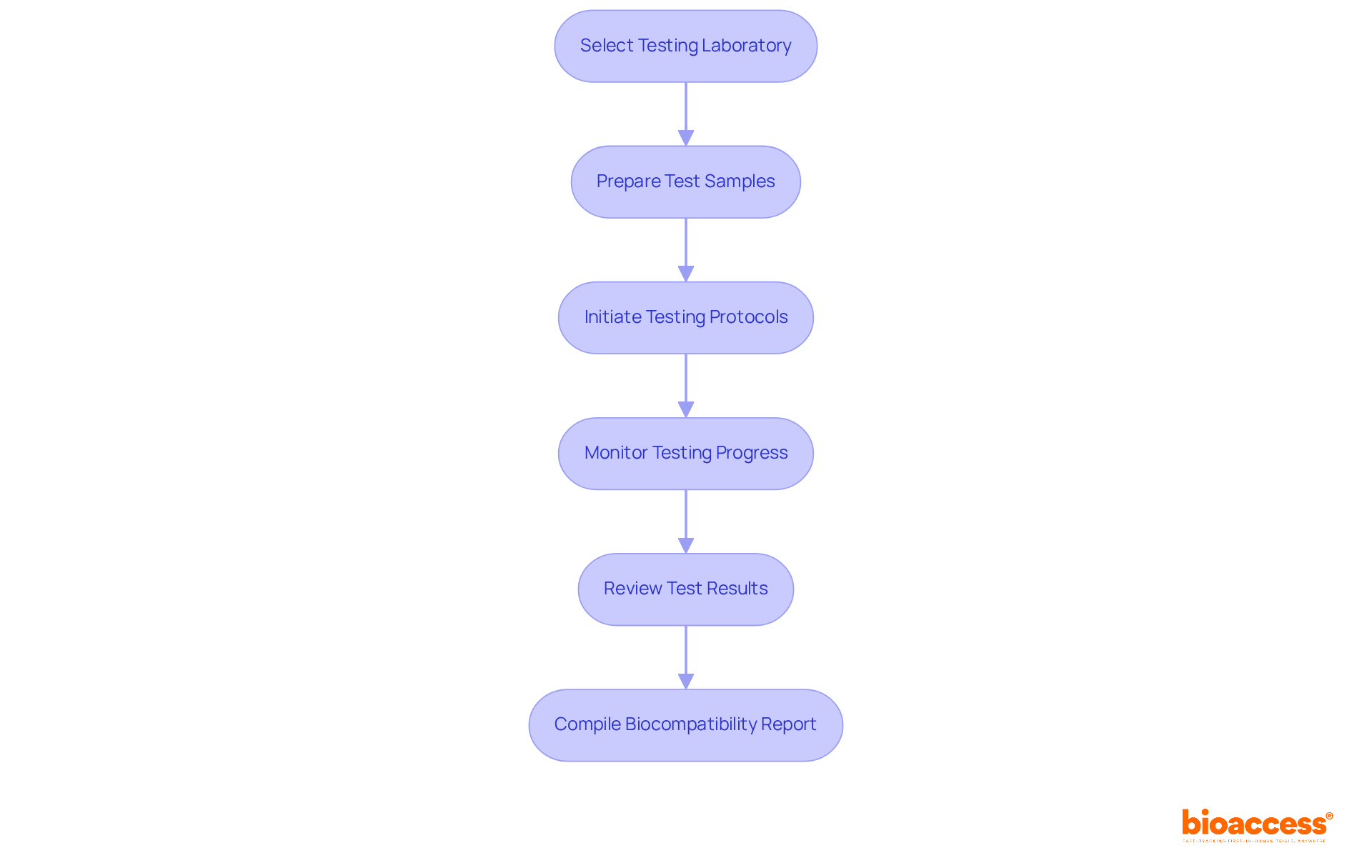
Conduct Biocompatibility Testing
To conduct biocompatibility testing for your medical device, it is essential to follow these structured steps:
- Select a testing laboratory that specializes in biocompatibility assessments and is accredited to perform the necessary evaluations according to ISO 10993 7.
- Prepare Test Samples: Ensure that the samples of your medical device are meticulously prepared according to the laboratory's specifications. This preparation may include sterilization and proper handling to avoid contamination.
- Initiate Testing Protocols: Collaborate with the laboratory to commence the examination protocols. It is crucial that all tests are conducted in accordance with the relevant ISO standards and that the laboratory adheres to Good Laboratory Practices (GLP).
- Monitor Testing Progress: Maintain proactive communication with the laboratory to observe the advancement of the evaluation. Address any issues that may arise during the evaluation phase promptly and effectively.
- Review Test Results: Once the evaluation is complete, review the results thoroughly. Ensure that the findings align with the biocompatibility requirements outlined in ISO 10993 7.
- Compile a Biocompatibility Report: Prepare a comprehensive report that includes the testing methodology, results, and conclusions. This report will be essential for regulatory submissions and demonstrating compliance.
Conclusion
Mastering ISO 10993-7 is crucial for ensuring the biocompatibility of medical devices, safeguarding patient health, and meeting regulatory standards. Understanding the fundamental principles of this standard and following the outlined steps enables professionals to navigate the complexities of biocompatibility compliance effectively.
This article details a comprehensive approach to mastering ISO 10993-7, which includes:
- Familiarization with key terms
- Assessing biocompatibility requirements
- Conducting rigorous testing
Each step—ranging from evaluating material composition to compiling thorough documentation—plays a vital role in achieving compliance and ensuring the safety of medical devices.
In summary, the importance of adhering to ISO 10993-7 cannot be overstated. As the medical device industry continues to evolve, it is vital to stay informed and compliant with biocompatibility standards. Engaging with industry resources and seeking expert guidance can further enhance understanding and implementation, ultimately leading to safer medical innovations.
Frequently Asked Questions
What is ISO 10993-7?
ISO 10993-7 is a standard within the ISO 10993 series that focuses on the biological assessment of medical instruments, specifically outlining the requirements for evaluating the suitability of materials used in medical devices.
Why is it important to understand ISO 10993-7?
Understanding ISO 10993-7 is crucial for ensuring that materials used in medical devices are safe and biocompatible, which is essential for the health and safety of patients.
How can one master ISO 10993-7?
To master ISO 10993-7, one should obtain a copy of the standard, familiarize themselves with its scope and definitions, identify key terms, review the biological evaluation process, study relevant guidelines, and engage with industry resources.
What key terms should be understood when studying ISO 10993-7?
Key terms include 'biocompatibility', 'cytotoxicity', and 'material characterization', which are essential for effective communication and implementation of the standard.
What steps are involved in the biological evaluation process according to ISO 10993-7?
The biological evaluation process involves risk assessment, testing, and documentation requirements.
Which related guidelines should be studied alongside ISO 10993-7?
Related guidelines include ISO 14971 (risk management) and ISO 10993-1 (general principles), as they provide important context and additional requirements for compatibility assessments.
How can one deepen their understanding of ISO 10993-7?
One can deepen their understanding by utilizing webinars, workshops, and industry publications that focus on ISO 10993-7 and its real-world applications.
List of Sources





