


The article underscores vital compliance strategies for clinical trials in accordance with MDCG 2020-6, highlighting the critical role of comprehensive medical data and stringent regulatory adherence. It delineates essential practices, including:
These strategies not only uphold regulatory standards but also enhance the integrity of clinical research, thereby reinforcing the necessity for a robust compliance framework.
Navigating the complexities of medical device regulations is a formidable challenge, particularly with the introduction of MDCG 2020-6, which imposes stringent compliance requirements on legacy devices. This article explores essential strategies for ensuring adherence to these guidelines, offering insights that can significantly enhance the integrity of clinical trials.
As organizations endeavor to meet these new standards, they frequently face challenges that prompt critical inquiries:
Addressing these issues is crucial for any organization aiming to thrive in the evolving landscape of medical device trials.
The document mdcg 2020-6 delineates the essential criteria for demonstrating adequate research for legacy medical devices under the new Medical Device Regulation (MDR). It underscores the critical need for comprehensive medical data to ensure safety and performance, particularly for devices that were previously CE marked under older directives. The key objectives encompass:
Understanding these principles is paramount for ensuring compliance and facilitating more streamlined regulatory processes in research trials. With over 20 years of expertise in Medtech, bioaccess® offers extensive trial management services, including:
to assist you in efficiently navigating these requirements. Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, provides invaluable insights into aligning your studies with MDCG 2020-6, ensuring that your evidence meets the necessary standards.
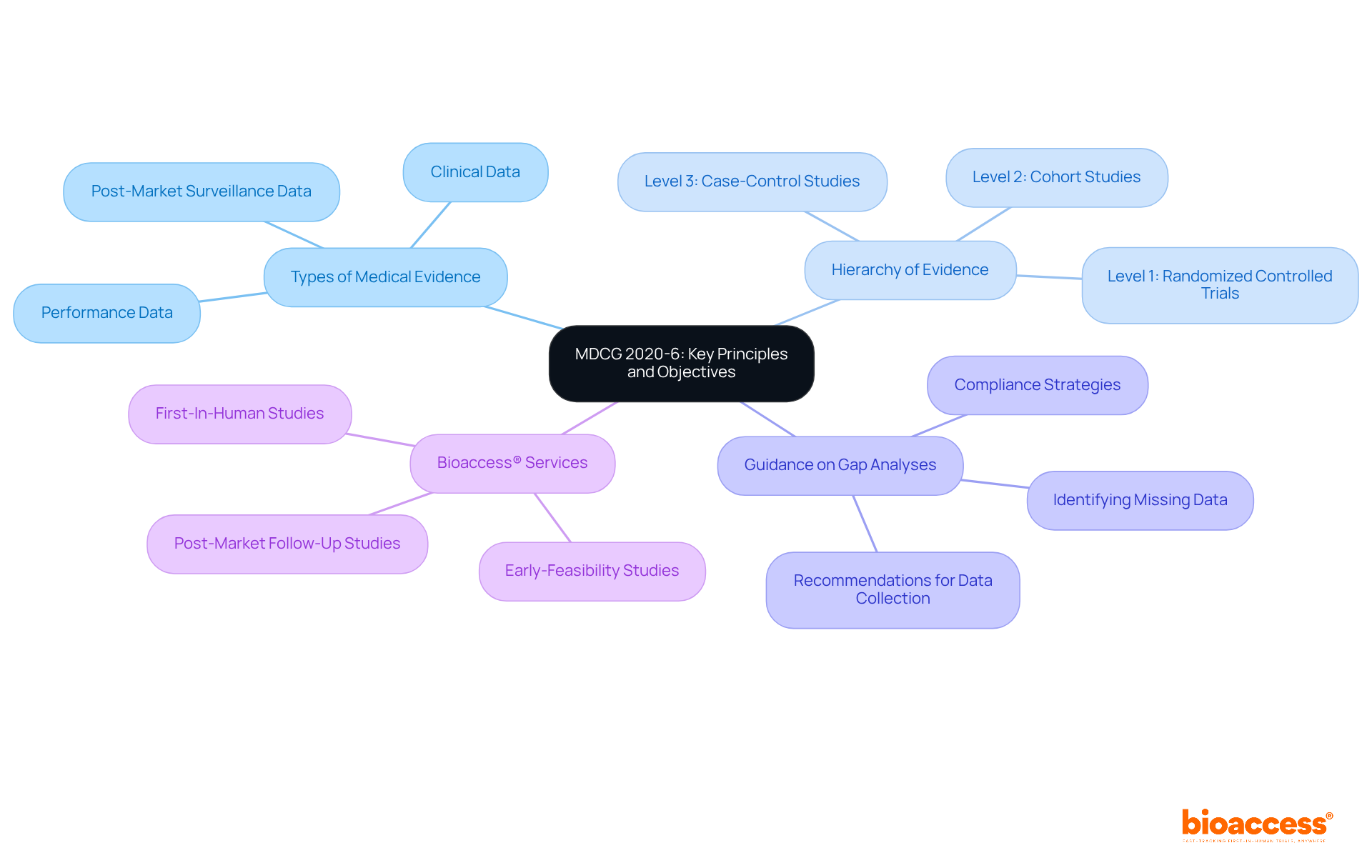
To implement effective compliance strategies, organizations must begin with a thorough review of existing medical data in relation to the requirements outlined in the guidelines of mdcg 2020-6. This foundational step is crucial for ensuring regulatory adherence and enhancing clinical research integrity.
Gap Analysis: Conduct a comprehensive assessment to identify any deficiencies in medical data. Develop a strategic plan to address these gaps, which may involve additional studies or targeted data collection efforts.
Documentation: It is essential to maintain meticulous records of all clinical evaluations. Ensure that these records align with the hierarchy of evidence as specified in the guidance, thereby reinforcing the credibility of the data collected.
Stakeholder Engagement: Foster transparent communication with regulatory bodies, ethics committees, and other relevant parties. This engagement is vital for ensuring consensus on adherence expectations and maintaining a collaborative approach to compliance.
Continuous Monitoring: Establish a robust system for ongoing adherence checks throughout the trial lifecycle. This proactive measure allows for timely adjustments as necessary, thereby enhancing the overall compliance framework.
By adopting these strategies, organizations can significantly improve their regulatory standing and facilitate smoother interactions with authorities, ultimately advancing their clinical research objectives.
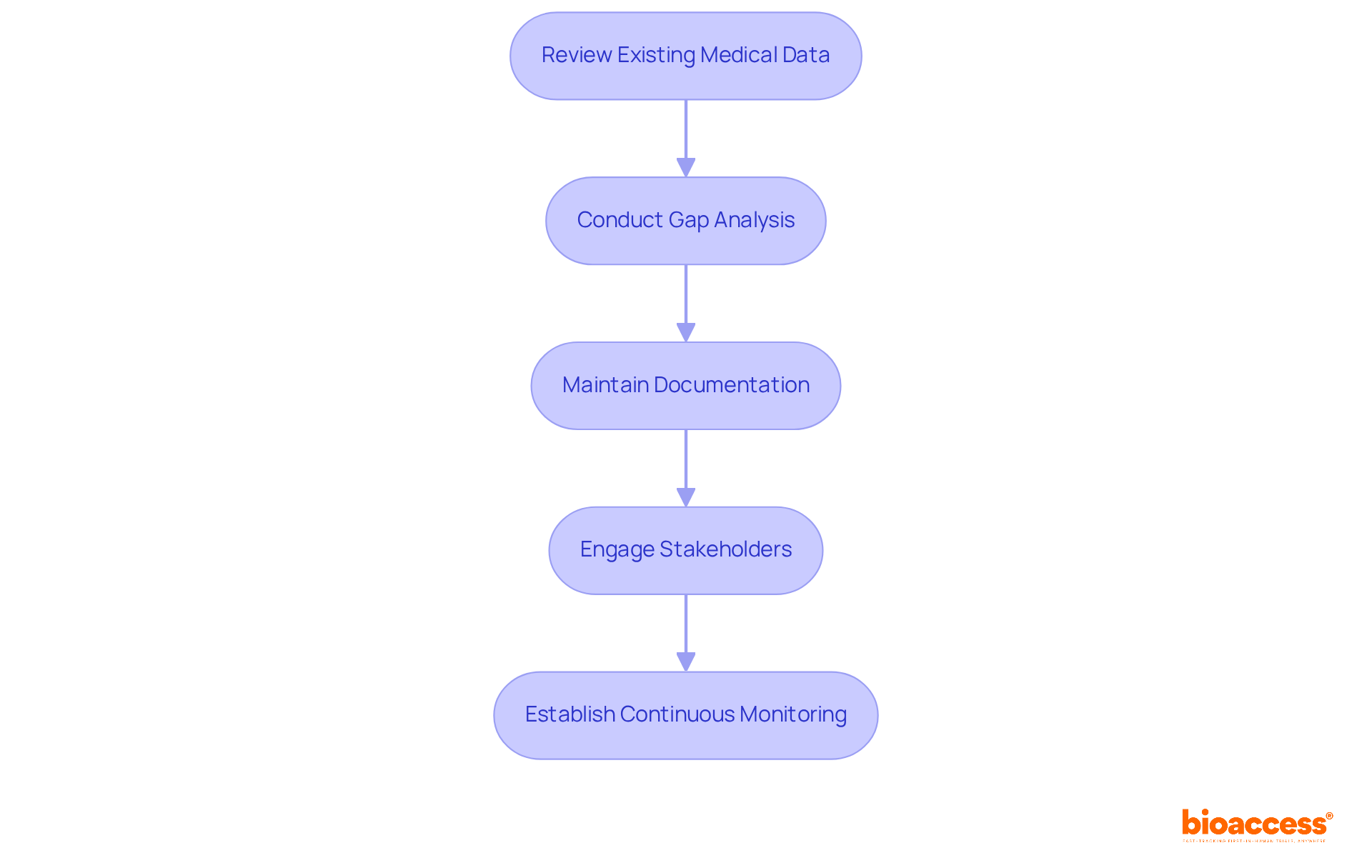
Monitoring compliance throughout the research lifecycle is critical for maintaining the integrity of clinical trials. Key practices include:
By incorporating these practices, organizations can sustain a high standard of adherence and ensure the integrity of their research trials.
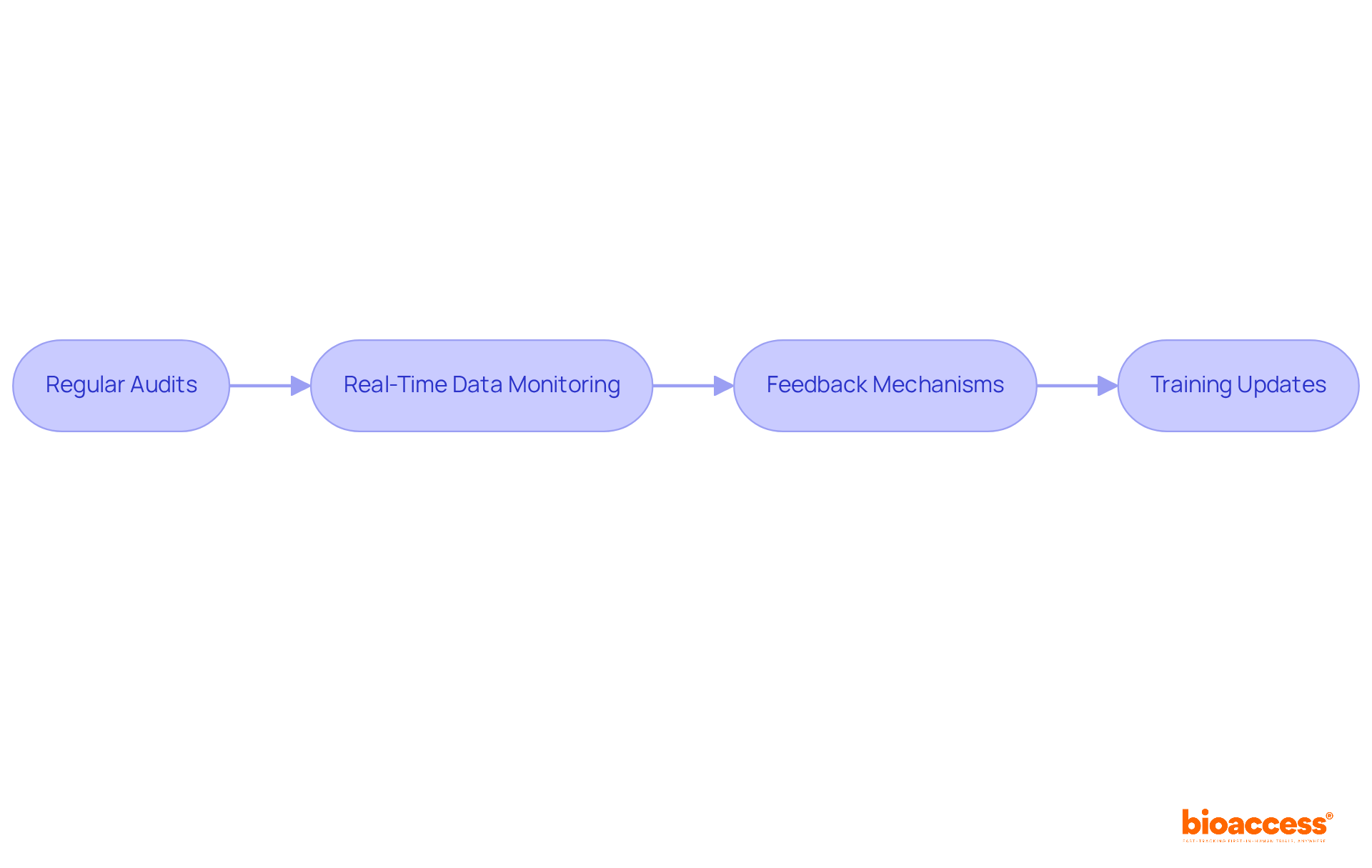
Training research teams on mdcg 2020-6 compliance requirements is essential for ensuring successful clinical trials. This process should encompass several key components:
By investing in thorough training, organizations empower their research teams to effectively navigate compliance challenges and contribute to successful clinical trials.
Mastering MDCG 2020-6 is essential for organizations involved in clinical trials, particularly those focused on legacy medical devices. This guidance not only outlines the necessary evidence required for compliance but also emphasizes the importance of a structured approach to data collection and regulatory adherence. By understanding and implementing the principles of MDCG 2020-6, organizations can significantly enhance their research integrity and ensure the safety and performance of their devices.
The article elaborates on several key strategies for achieving compliance, including:
Training research teams on these compliance requirements is equally vital, as it empowers them to navigate regulatory challenges and fosters a culture of adherence throughout the research lifecycle. Each of these strategies plays a critical role in aligning clinical trials with the rigorous standards set forth by MDCG 2020-6.
Ultimately, the significance of adhering to MDCG 2020-6 cannot be overstated. Organizations must prioritize the implementation of these compliance strategies to not only meet regulatory expectations but also to contribute to the advancement of safe and effective medical devices in the market. Embracing these practices will not only streamline the regulatory process but also enhance the overall quality of clinical research, paving the way for innovations that can positively impact patient outcomes.
What is MDCG 2020-6?
MDCG 2020-6 is a document that outlines the essential criteria for demonstrating adequate research for legacy medical devices under the new Medical Device Regulation (MDR).
Why is MDCG 2020-6 important?
It is important because it emphasizes the need for comprehensive medical data to ensure the safety and performance of medical devices, particularly those that were previously CE marked under older directives.
What are the key objectives of MDCG 2020-6?
The key objectives include clarifying the types of medical evidence required, establishing a hierarchy of evidence, and guiding manufacturers in conducting gap analyses to identify any missing data.
How can understanding MDCG 2020-6 benefit manufacturers?
Understanding MDCG 2020-6 is crucial for ensuring compliance with regulations and facilitating more streamlined regulatory processes in research trials.
What services does bioaccess® offer related to MDCG 2020-6?
bioaccess® offers extensive trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Follow-Up Studies to help navigate the requirements of MDCG 2020-6.
Who provides insights into aligning studies with MDCG 2020-6?
Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, provides insights into aligning studies with MDCG 2020-6 to ensure that evidence meets the necessary standards.