


This article serves as a comprehensive step-by-step guide to mastering medical device development, detailing crucial stages such as:
Each phase is vital for ensuring product safety, efficacy, and compliance. The emphasis on rigorous testing, user research, and continuous improvement processes underscores their critical role in successful market introduction and ongoing product performance. By understanding these key stages, stakeholders can navigate the complexities of the Medtech landscape effectively.
Navigating the intricate landscape of medical device development poses significant challenges for innovators in the Medtech, Biopharma, and Radiopharma sectors. This comprehensive guide serves to demystify the essential stages of the development process, encompassing everything from initial concept and feasibility studies to post-market surveillance. By offering valuable insights, it aims to significantly enhance the chances of success.
As the industry evolves and regulatory landscapes shift, developers must ask themselves:
The medical device development process encompasses several critical stages that ensure a successful product launch:
Concept and Feasibility: This foundational phase involves generating ideas, analyzing market needs, and assessing the feasibility of the proposed device. Conducting preliminary research is essential to validate the concept and align it with market demands. Leveraging comprehensive clinical trial management services, such as those offered by bioaccess®, enhances the feasibility assessment through expert site selection and compliance reviews.
Creation and Development: During this phase, detailed plans are crafted, and prototypes are made. This includes setting engineering specifications and performing initial testing to verify that the layout meets user requirements.
Verification and Validation: Rigorous testing is conducted to verify that the apparatus adheres to design specifications and validate its intended purpose. This phase often includes preclinical studies and clinical trials, which are crucial for ensuring safety and efficacy. Bioaccess® specializes in overseeing different kinds of clinical studies, including Early-Feasibility and First-In-Human trials, which are essential for validating new medical instruments.
Regulatory Approval: Preparation and submission of documentation to regulatory bodies, such as the FDA, are vital for obtaining approval. This process requires demonstrating compliance with established safety and efficacy standards, which can take an average of five months for FDA 510(k) applications. Understanding the regulatory landscape, including the role of INVIMA as a Level 4 health authority in Colombia, is crucial for navigating approvals in Latin America.
Market Launch: Following approval, the product is ready for market introduction. This stage involves scaling production, planning distribution, and implementing marketing strategies to effectively reach target audiences.
Post-Market Surveillance: Continuous monitoring of the product's performance in the market is essential to ensure ongoing safety and effectiveness. This stage helps identify any potential issues and informs necessary adjustments to maintain compliance and user satisfaction.
Grasping these phases is essential for Medtech, Biopharma, and Radiopharma innovators seeking to navigate the intricacies of medical device development successfully. Highlighting the significance of the concept and feasibility stages, along with the obstacles encountered by healthcare startups, can greatly impact the overall success of the development process.

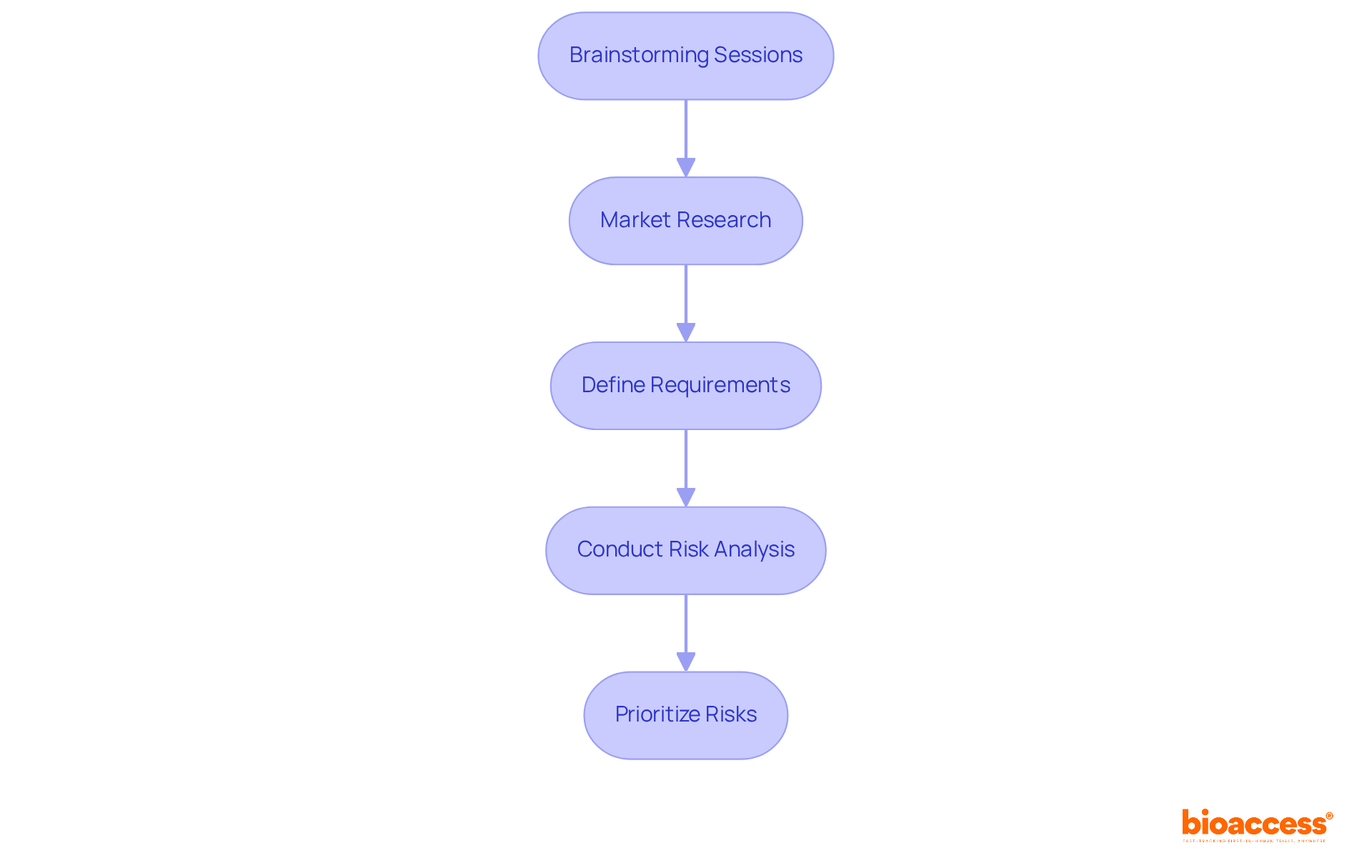
To effectively initiate ideation and risk analysis in medical device development, it is essential to follow these structured steps:
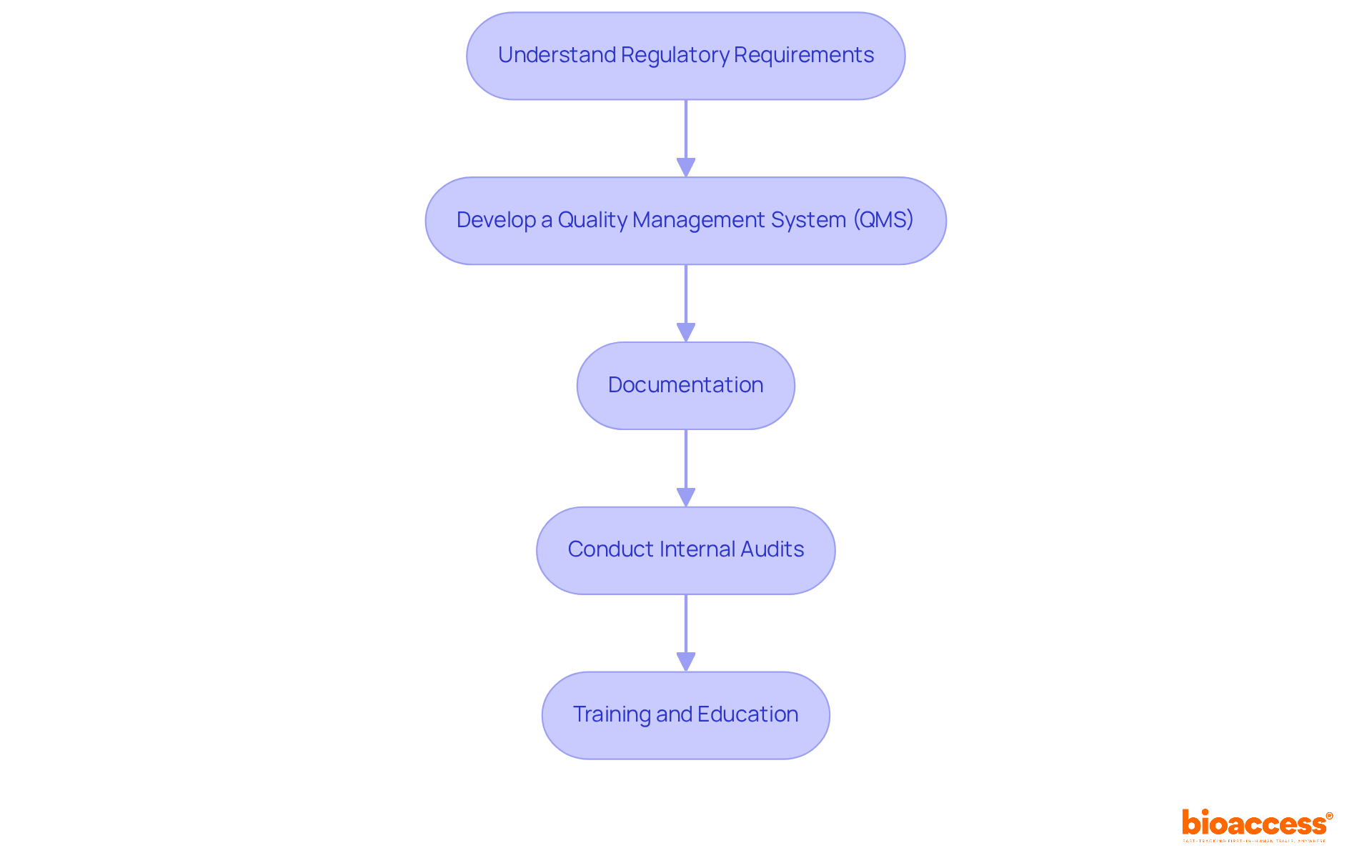
To ensure regulatory compliance and effective quality management in medical device development, it is essential to follow these key steps:
Understand Regulatory Requirements: Familiarize yourself with applicable regulations, such as FDA guidelines and ISO 13485 standards. This includes understanding equipment classification, premarket submissions, and post-market responsibilities, which are vital for compliance.
Develop a Quality Management System (QMS): Establish a robust QMS that documents all processes, procedures, and responsibilities necessary to achieve quality objectives. Aligning your QMS with regulatory standards in medical device development facilitates compliance and enhances operational efficiency.
Documentation: Maintain comprehensive documentation throughout the development process, including design history files, risk management files, and validation reports. This documentation is crucial for regulatory submissions in medical device development and demonstrates adherence to quality standards.
Conduct Internal Audits: Regularly review processes and compliance with the QMS. Internal audits help identify areas for improvement and ensure that corrective actions are implemented effectively, fostering a culture of continuous improvement.
Training and Education: Provide ongoing training for team members on regulatory requirements and quality management practices. Ensuring that all staff are aligned with compliance goals enhances the overall effectiveness of the QMS and reduces the risk of non-compliance.
Executing these actions not only fulfills regulatory standards but also enhances the overall quality and safety of healthcare products, ultimately establishing trust with clients and stakeholders.
Integrating participant research and human factors testing is crucial for medical device development aimed at creating effective medical devices. This user-centered approach can significantly enhance product outcomes in medical device development, ensuring that the devices meet the actual needs of users. Follow these steps to implement this methodology effectively:
Identify Individual Needs: Engage potential participants through interviews, surveys, or focus groups to gather insights into their needs, preferences, and pain points. This essential step is vital, as comprehending client needs can greatly impact the results of medical device development.
Develop Audience Personas: Create detailed audience personas that encapsulate the target demographic. These personas assist in comprehending individual behaviors and expectations, informing choices that resonate with real users.
Usability Testing: Conduct usability tests with prototypes to observe user interactions with the product. This phase is crucial for collecting feedback on usability, functionality, and appearance. For instance, a study involving 140 participants evaluated an anaphylactic shock auto-injector, underscoring the importance of testing under varying fidelity conditions to detect use errors effectively. Additionally, rigorous usability assessments are mandatory for medical device development to identify potentially harmful use errors, ensuring compliance with regulatory standards.
Iterate Based on Feedback: Utilize insights from usability testing to enhance the product's layout. Repetitive modifications based on feedback can improve user experience and safety, ultimately leading to better health outcomes. Ongoing attention to individuals can drive advancements in healthcare technology, particularly in medical device development, as evidenced by the notable increase in minimally invasive robotic surgical tools that emphasize user-focused development. As DeviceLab states, 'User research is integral to outstanding medical device development.'
Document Findings: Maintain detailed records of user research and testing outcomes. This documentation is essential not only for regulatory submissions but also serves as a valuable resource for future design iterations. By prioritizing human factors testing, Medtech innovators can significantly improve product usability, reduce the risk of errors, and enhance overall patient safety, aligning with the industry's projected growth to $678.88 billion by 2025, with a growth rate expected to reach 6.1% per year from 2021 onwards.

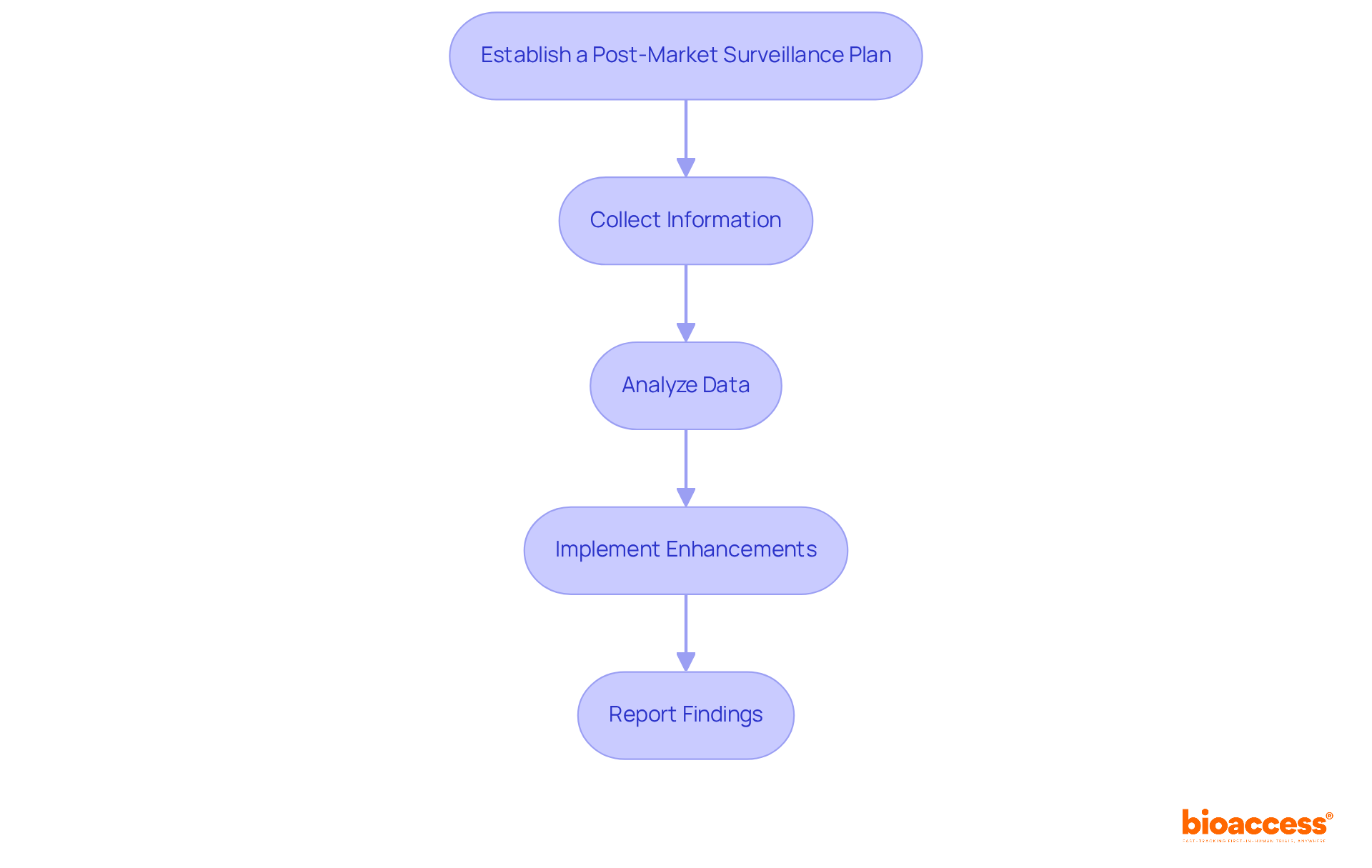
To effectively conduct post-market surveillance and foster continuous improvement, it is essential to follow these steps:
Integrating ongoing enhancement methods is crucial for improving safety in medical device development. For instance, nearly a third of medical equipment adverse event reports sent to the FDA were delayed, often exceeding six months after the producer was notified. This underscores the importance of timely reporting and proactive monitoring to ensure patient safety and device efficacy. By implementing a robust post-market surveillance strategy, manufacturers involved in medical device development can not only comply with regulatory requirements but also significantly enhance their products and processes over time.
Mastering the medical device development process is essential for innovators aiming to create effective and compliant healthcare solutions. This article serves as a comprehensive guide navigating the critical stages—from initial concept and feasibility to post-market surveillance. Each phase is meticulously designed to ensure that medical devices not only meet regulatory standards but also address user needs, ultimately enhancing patient safety and satisfaction.
Key arguments underscore the significance of:
By integrating user research and human factors testing, developers can create products that resonate with actual user needs, significantly reducing the risk of errors and improving usability. Furthermore, establishing a robust post-market surveillance strategy facilitates continuous improvement, ensuring that devices remain safe and effective long after their launch.
In conclusion, the medical device development lifecycle is a multifaceted journey that demands careful planning, adherence to regulations, and a commitment to user-centered design. As the industry evolves, embracing these structured methodologies will enhance the quality of medical devices and drive innovation in healthcare technology. Stakeholders are encouraged to prioritize these steps to foster a safer, more effective medical device landscape, ultimately contributing to better health outcomes for all.
What are the key stages of medical device development?
The key stages of medical device development include Concept and Feasibility, Creation and Development, Verification and Validation, Regulatory Approval, Market Launch, and Post-Market Surveillance.
What is involved in the Concept and Feasibility stage?
This stage involves generating ideas, analyzing market needs, and assessing the feasibility of the proposed device. Preliminary research is conducted to validate the concept and ensure it aligns with market demands.
What happens during the Creation and Development phase?
In this phase, detailed plans are crafted, prototypes are developed, engineering specifications are set, and initial testing is performed to verify that the design meets user requirements.
What is the purpose of the Verification and Validation stage?
This stage involves rigorous testing to ensure the device adheres to design specifications and validates its intended purpose, including preclinical studies and clinical trials to confirm safety and efficacy.
What is required for Regulatory Approval?
This involves preparing and submitting documentation to regulatory bodies like the FDA to demonstrate compliance with safety and efficacy standards. The FDA 510(k) application process typically takes about five months.
What occurs during the Market Launch stage?
After obtaining regulatory approval, the product is introduced to the market, which includes scaling production, planning distribution, and implementing marketing strategies to reach target audiences.
Why is Post-Market Surveillance important?
Continuous monitoring of the product's performance in the market is essential to ensure ongoing safety and effectiveness, helping to identify potential issues and inform necessary adjustments.
What steps should be followed to initiate ideation and risk analysis in medical device development?
Steps include conducting brainstorming sessions, performing market research, defining requirements, conducting risk analysis, and prioritizing risks to focus on high-priority challenges.
How can risk analysis be conducted?
Risk analysis can be conducted by identifying potential risks related to technical, regulatory, and market challenges, using tools like Failure Mode and Effects Analysis (FMEA) to evaluate these risks systematically.
What is the significance of understanding the concept and feasibility stages?
Understanding these stages is crucial for Medtech, Biopharma, and Radiopharma innovators as they navigate the complexities of medical device development, impacting the overall success of the process.