


Navigating the complex landscape of medical device registration in Mexico is no small feat for manufacturers, especially considering the pivotal role of COFEPRIS, the nation's regulatory authority. Understanding this intricate process is crucial - not only for ensuring compliance but also for tapping into a rapidly growing market projected to reach USD 9.72 billion by 2028.
With regulations constantly evolving and a multi-step registration process in place, how can companies streamline their entry into this competitive arena? This guide explores the essential steps, challenges, and documentation requirements necessary for successful medical device registration in Mexico, equipping manufacturers with the knowledge they need to thrive.
The Federal Commission for Protection against Sanitary Risk (COFEPRIS) serves as the regulatory authority overseeing medical instruments in Mexico. Operating under the Ministry of Health, COFEPRIS is essential for ensuring the safety, effectiveness, and quality of health-related products. For producers aiming for medical device registration in Mexico, understanding this regulatory body is crucial.
Familiarity with COFEPRIS's classification system is vital, as it categorizes medical equipment into three distinct risk levels:
Each class comes with specific regulatory requirements and approval timelines. Notably, Class I devices typically undergo quicker review processes compared to their higher-risk counterparts, which can significantly impact market entry strategies.
Recent reforms have streamlined the enrollment system, leading to a notable reduction in approval timelines. For instance, while the assessment period for health equipment approval is projected to range from 3 to 8 months in 2025, involving an Authorized Third Party can expedite this process to just 1 to 3 months. Staying informed about these regulatory changes is essential for manufacturers, enabling them to navigate the medical device registration in Mexico process more efficiently and take advantage of the burgeoning medical equipment market in Mexico, which is expected to reach USD 9.72 billion by 2028.

The registration process for medical devices in Mexico involves several essential steps that are crucial for successful market entry:
Device Classification: Start by determining the classification of your medical device - Class I, II, or III - based on its intended use and associated risk level. This classification is vital as it dictates the subsequent steps and documentation requirements.
Appoint a Mexico Registration Holder (MRH): For foreign manufacturers, appointing a local representative or MRH is mandatory. This individual will represent you in all dealings with the regulatory authority, ensuring compliance with local regulations.
Prepare the Submission Dossier: Compile a comprehensive submission dossier that includes technical documentation, clinical evaluation reports, and proof of compliance with Good Manufacturing Practices (GMP). This dossier must be meticulously organized to meet regulatory standards.
Submit Application: Submit your application along with the registration dossier to the relevant authority. Ensure that all documents are complete and tailored to the specific requirements of your classification.
Respond to Queries: Be prepared to address any inquiries or requests for additional information from COFEPRIS during the evaluation. Timely and thorough responses are critical to maintaining momentum in your application.
Receive Approval: Upon acceptance, you will obtain a certification, allowing you to market your device in Mexico. Keep in mind that enrollments are valid for five years and require renewal before expiration, necessitating proactive management of the renewal.
The significance of appointing a Mexico Registration Holder in the context of medical device registration in Mexico cannot be overstated. Industry specialists emphasize that having an informed local representative is essential for navigating the complexities of the registration process and ensuring adherence to regulatory standards. This strategic partnership can significantly enhance your chances of successful market entry in Mexico.

To successfully register your medical device with COFEPRIS, it’s essential to prepare the following documentation:
Application Form: Complete the official application form provided by COFEPRIS. Ensure all sections are filled out accurately to avoid delays in processing.
Certificate of Free Sale (CFS): Obtain a CFS from the health authority in your country of origin. This certificate is crucial as it verifies that the equipment is legally sold in that market, ensuring compliance with international trade regulations. Without a CFS, manufacturers may encounter significant supply chain issues, including shipment delays or customs rejections.
Quality Management System Documentation: Provide evidence of compliance with ISO 13485 or equivalent quality management standards. This includes audit reports that demonstrate adherence to quality protocols, which are vital for regulatory approval.
Clinical Evaluation Report: Include a report that demonstrates the clinical safety and efficacy of your product, based on clinical data or literature. This report is critical, as approximately 15% of medical equipment applications are rejected due to insufficient documentation, underscoring the importance of comprehensive clinical evidence.
Technical Documentation: Prepare detailed technical specifications, labeling information, and a risk analysis for your equipment. This documentation must clearly outline how the device meets safety and performance standards.
Manufacturing Facility Information: Submit details about the manufacturing facility, including any relevant certifications and inspection reports. This information assures the regulatory agency of the facility's compliance with local regulations.
Ensure that all documents are translated into Spanish and formatted according to COFEPRIS requirements to facilitate a smooth review. By meticulously preparing these documents, you can significantly enhance the chances of a successful application for medical device registration in Mexico and expedite your entry into the Mexican market.

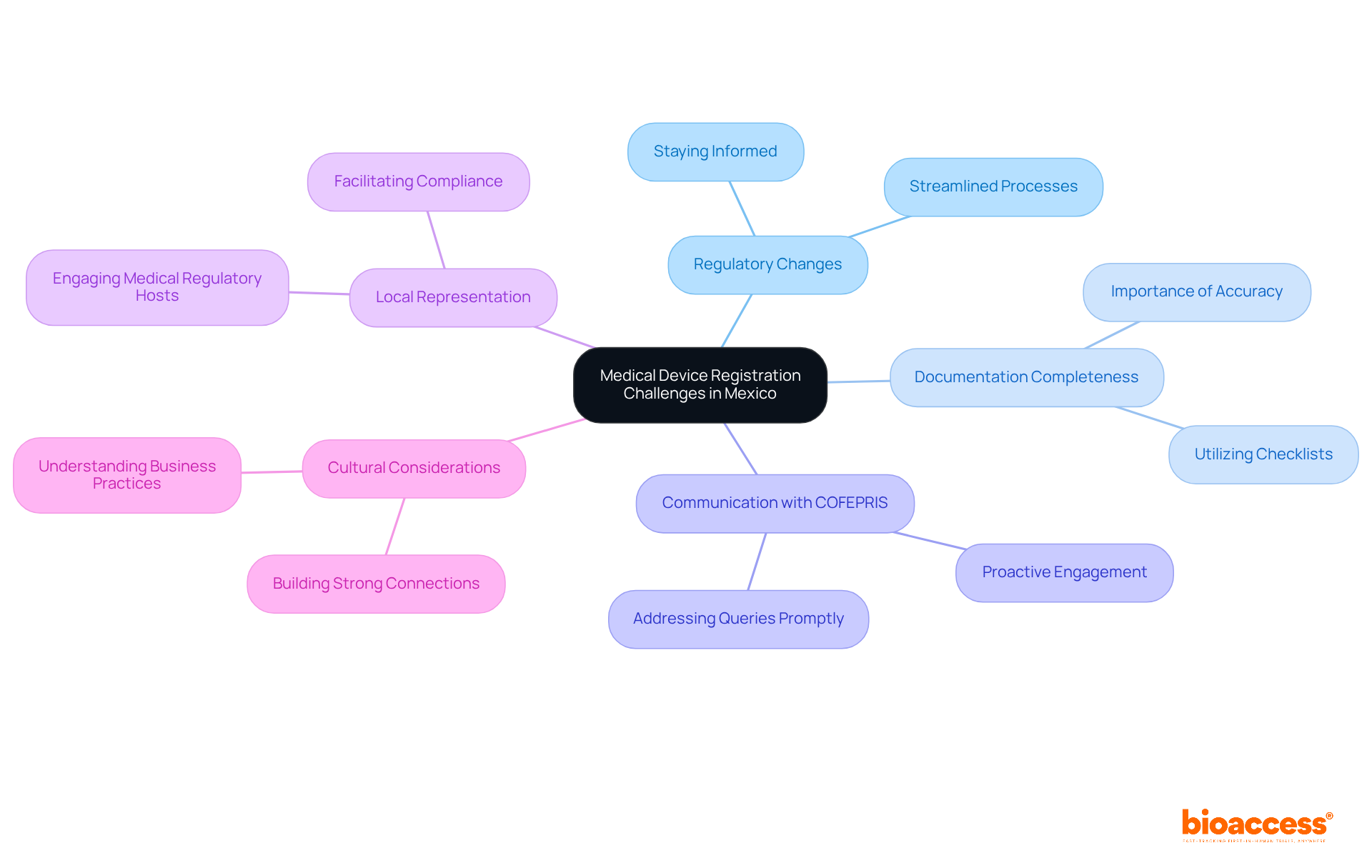
Navigating the medical device registration Mexico process presents several challenges that demand careful consideration. Understanding these key issues is essential for success:
Regulatory Changes: Staying informed about health authority regulations is crucial. Recent adjustments have streamlined the registration process, making it more accessible for manufacturers. Regularly consulting official communications and industry updates from the health authority will keep you informed.
Documentation Completeness: The accuracy and completeness of your documentation are vital. Incomplete submissions can lead to significant delays or outright rejections. Utilizing a detailed checklist ensures that all necessary documents are included, enhancing your chances of a successful application.
Communication with COFEPRIS: Open communication with COFEPRIS is essential. Promptly addressing any queries or requests for additional information can prevent unnecessary prolongation of the review process. This proactive approach can significantly shorten enrollment timelines.
Local Representation: For foreign manufacturers, engaging a reliable Medical Regulatory Host (MRH) is crucial. An experienced MRH can facilitate compliance with local regulations and enhance communication with COFEPRIS, smoothing the path to market entry.
Cultural Considerations: Understanding cultural nuances in business practices and communication styles can greatly benefit your interactions with local stakeholders. Establishing strong connections encourages teamwork and assistance throughout the enrollment procedure.
By anticipating these challenges and preparing strategically, you can effectively streamline your medical device registration Mexico process. This approach allows you to capitalize on the favorable regulatory environment and growing market demand.
Understanding the complexities of medical device registration in Mexico is crucial for manufacturers aiming to tap into this lucrative market. By recognizing the pivotal role of COFEPRIS and adhering to its guidelines, businesses can effectively navigate compliance challenges and ensure their products meet safety and efficacy standards. While the registration process is detailed and rigorous, it can be managed systematically, highlighting the necessity of local representation and thorough documentation.
Key steps in the registration journey include:
Each stage is vital for securing timely approval from COFEPRIS, particularly as regulatory reforms continue to evolve. Moreover, addressing challenges such as documentation completeness and maintaining open communication with regulatory authorities can significantly enhance the chances of successful market entry.
Ultimately, the increasing demand for medical devices in Mexico presents an exciting opportunity for manufacturers. By mastering the registration process and staying informed about regulatory changes, businesses can position themselves for success in this dynamic environment. Taking proactive measures to understand and comply with COFEPRIS guidelines will not only facilitate smoother registration but also contribute to the overall health and safety of the Mexican healthcare landscape.
What is COFEPRIS?
COFEPRIS, the Federal Commission for Protection against Sanitary Risk, is the regulatory authority in Mexico that oversees the safety, effectiveness, and quality of medical devices and health-related products.
What role does COFEPRIS play in medical device registration?
COFEPRIS is essential for ensuring that medical devices meet regulatory requirements for safety and effectiveness, which is crucial for producers seeking to register their products in Mexico.
How are medical devices classified by COFEPRIS?
COFEPRIS classifies medical devices into three risk levels: Class I (low risk), Class II (moderate risk), and Class III (high risk). Each class has specific regulatory requirements and approval timelines.
What are the approval timelines for different classes of medical devices?
Class I devices usually have quicker review processes, while Class II and Class III devices take longer. The assessment period for health equipment approval is projected to be between 3 to 8 months in 2025, but involving an Authorized Third Party can expedite this to 1 to 3 months.
What recent changes have been made to the COFEPRIS enrollment system?
Recent reforms have streamlined the enrollment system, leading to a reduction in approval timelines for medical devices.
Why is it important for manufacturers to stay informed about COFEPRIS regulations?
Staying informed about COFEPRIS regulations enables manufacturers to navigate the medical device registration process more efficiently and capitalize on the growing medical equipment market in Mexico, which is projected to reach USD 9.72 billion by 2028.