


The article delineates five actionable steps essential for mastering medical device compliance. It underscores the significance of:
Each step is bolstered by detailed explanations and examples, such as the critical need for risk management and quality management systems. These elements are indispensable for ensuring the safety and efficacy of medical devices, ultimately facilitating successful market outcomes.
Mastering medical device compliance is not merely a regulatory requirement; it is a critical component of ensuring patient safety and product efficacy in a complex and evolving healthcare landscape. By delving into the fundamentals of compliance, organizations can unlock a wealth of knowledge that enhances their operational strategies and market success.
However, with a myriad of regulations and standards to navigate, how can companies effectively streamline their compliance processes and avoid the pitfalls that lead to costly mistakes? This question is essential for organizations aiming to thrive in the Medtech sector, where the stakes are high and the consequences of non-compliance can be severe.
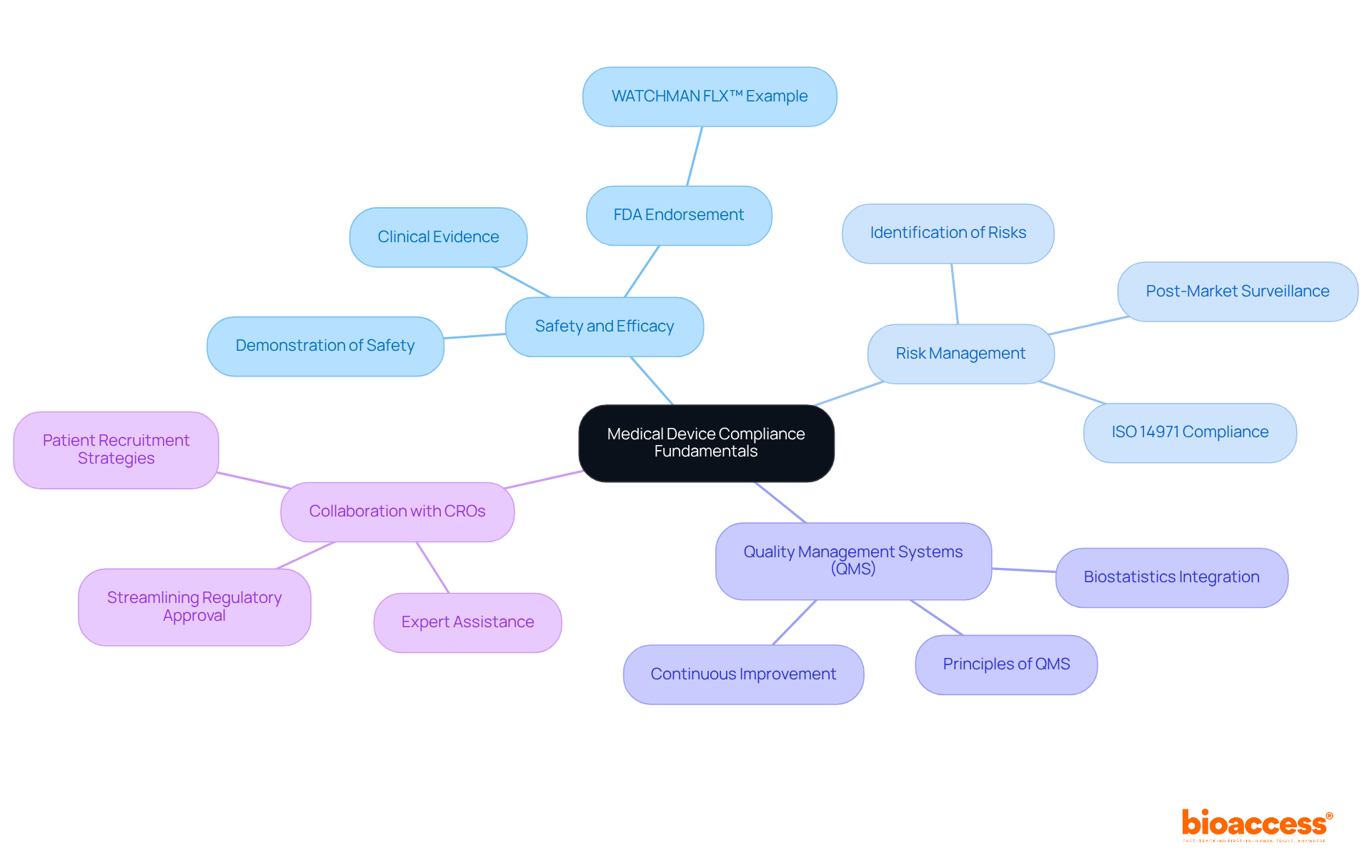
Mastering medical devices compliance is paramount, beginning with a solid understanding of its core principles that are essential for ensuring the safety and efficacy of medical tools. Key concepts include:
Safety and Efficacy: Medical devices must demonstrate safety for users and effectiveness for their intended purposes. The FDA's endorsement of Boston Scientific’s WATCHMAN FLX™ product illustrates how rigorous testing can lead to innovative solutions for stroke prevention in atrial fibrillation patients. This example showcases the importance of establishing safety and efficacy through robust clinical evidence.
Risk Management: Identifying potential risks linked to a piece of equipment is crucial. Effective risk management practices involve systematic documentation and adherence to standards such as ISO 14971, guiding manufacturers in assessing and mitigating risks throughout the product lifecycle. Continuous monitoring and post-market surveillance are vital for ensuring the safety and efficacy of devices, which is essential for medical devices compliance, as evidenced by the financial and reputational impacts of non-compliance highlighted in various case studies.
Quality Management Systems (QMS): Familiarity with QMS principles is essential for maintaining product quality. A well-implemented QMS ensures that processes are in place to uphold quality standards from design through to post-market evaluation. Companies that incorporate biostatistics early in their development process often attain improved clinical results and compliance assurance, reinforcing the significance of a thorough approach to quality management.
In addition to these fundamentals, collaborating with a specialized contract research organization (CRO) like bioaccess® can significantly enhance your adherence journey. With expertise in accelerating clinical trials for Medtech, Biopharma, and Radiopharma startups, bioaccess® offers tailored solutions that streamline regulatory approval, clinical research site activation, and patient recruitment. By utilizing bioaccess®'s services, including assistance with fundraising and attaining successful exits, you can manage the intricacies of regulations more efficiently and progress your healthcare product from pilot study to commercialization with confidence.
By mastering these fundamentals and leveraging expert assistance, you will be better prepared to navigate the complexities of medical devices compliance in the healthcare equipment sector. This ultimately contributes to enhanced patient outcomes and market success.
Recognizing the essential regulatory frameworks and standards relevant to your healthcare product is vital for ensuring medical devices compliance and achieving market success. Here are the primary frameworks to consider:
FDA Regulations (USA): The Food and Drug Administration (FDA) oversees and regulates medical devices in the United States. Familiarity with 21 CFR Part 820, which outlines the Quality System Regulation (QSR), is essential. Current data shows that the FDA oversees more than 190,000 unique medical instruments, underscoring the necessity for medical devices compliance.
European Medical Device Regulation (EU MDR): For products marketed in Europe, understanding the EU MDR is essential. This regulation imposes stringent requirements for safety and performance, with recent updates clarifying key terms and the obligations for medical devices compliance. As of early 2025, manufacturers must inform authorities of anticipated supply disruptions for specific products, reflecting the regulation's emphasis on patient safety and market transparency.
ISO Standards: The International Organization for Standardization (ISO) offers essential guidelines for quality management systems related to medical equipment. ISO 13485 is particularly relevant, as it outlines the requirements for a comprehensive quality management system. Adherence to these standards not only aids in obtaining approval but also contributes to medical devices compliance, thereby improving product safety and effectiveness.
Investigating the particular rules that pertain to your equipment category and market is crucial for upholding standards and guaranteeing successful commercialization.

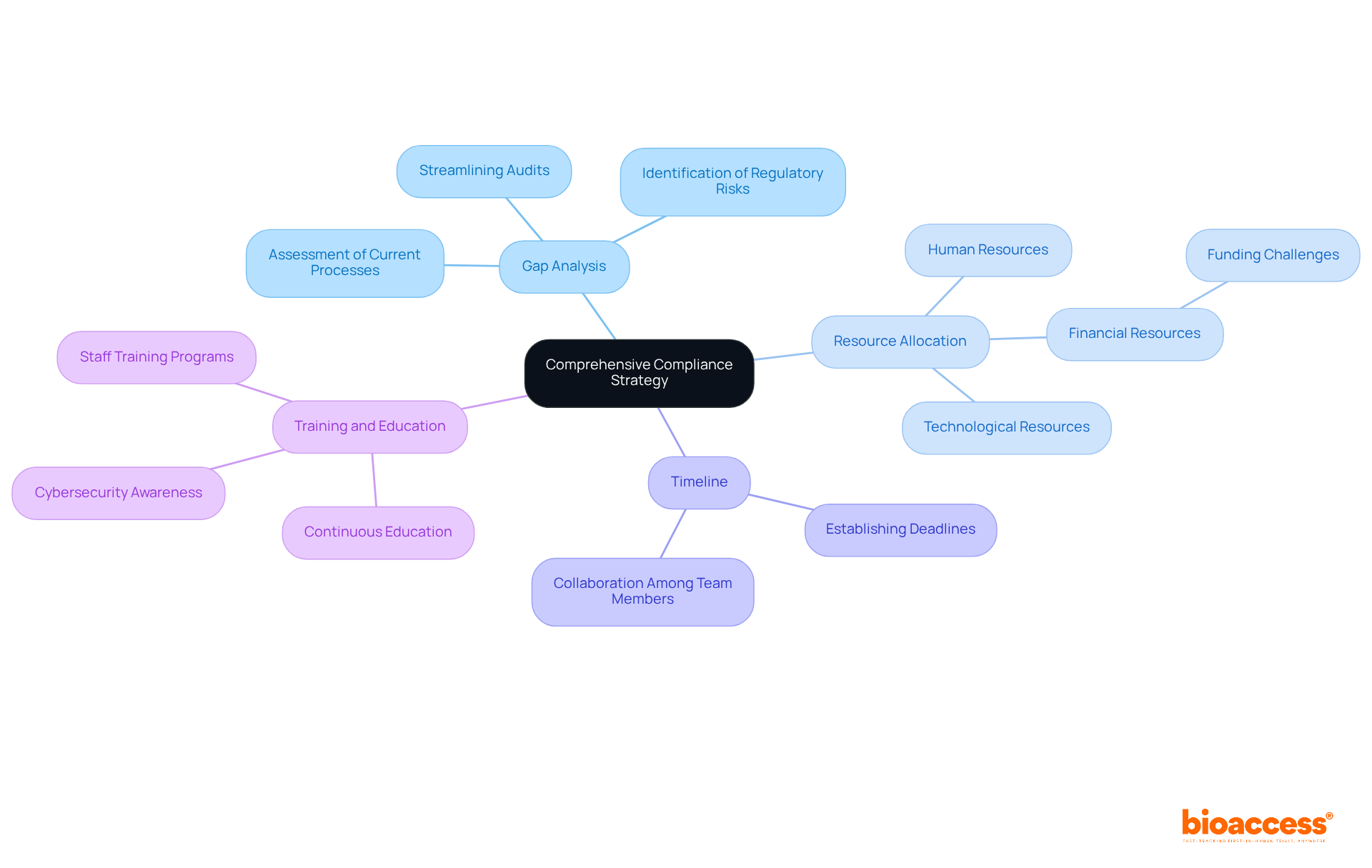
Navigating the complex landscape of medical device regulations necessitates the development of a comprehensive compliance strategy that incorporates several key components:
Gap Analysis: Conduct a thorough assessment of your current processes against regulatory requirements. This analysis is essential for pinpointing areas that require enhancement, serving as the foundation for your adherence initiatives. Frequent gap assessments can assist organizations in identifying regulatory risks early, streamline audits, and enhance the overall security posture.
Resource Allocation: Evaluate the necessary resources—human, financial, and technological—to effectively implement regulatory measures. Data indicates that 41% of healthcare providers believe they lack adequate funding for effective security strategies, underscoring the significance of strategic resource allocation in achieving adherence. Additionally, account for the complexities of trial setup, including obtaining necessary import permits and nationalizing investigational devices.
Timeline: Establish a clear timeline for achieving regulatory milestones. This ensures that all team members are aware of deadlines and can collaborate effectively towards meeting them. A well-structured timeline facilitates systematic progress and accountability.
Training and Education: Implement extensive training programs for staff to ensure they understand regulatory requirements and best practices. With nearly a third of healthcare staff unaware of their workplace's formal cybersecurity policy, continuous education is vital for fostering a culture of adherence and security awareness.
By developing a comprehensive strategy that includes these components, organizations can streamline their efforts and significantly enhance their likelihood of achieving successful adherence in the evolving regulatory environment. This proactive approach not only mitigates risks but also positions your organization for future success.
To effectively implement the compliance procedures and best practices identified in your strategy, focus on the following key actions:
Documentation: Ensure comprehensive documentation of all compliance-related activities, including risk assessments, training records, and quality control measures. This practice is essential, as organizations with effective documentation processes can reduce compliance-related risks by up to 60%. Additionally, knowledge workers spend an average of 50% of their time creating and preparing documents, highlighting the importance of efficient documentation practices.
Standard Operating Procedures (SOPs): Create and implement detailed SOPs that outline processes for adherence, ensuring consistency across the organization. SOPs not only streamline operations but also enhance communication among stakeholders, reducing misunderstandings and conflicts. Companies that implement SOPs report operational efficiency increases of up to 30%, and those with effective SOPs experience up to 25% lower turnover rates, underscoring the broader benefits of implementing these procedures.
Internal Audits: Conduct regular internal audits to evaluate adherence to established procedures and identify areas for improvement. These audits are essential for ensuring medical devices compliance with regulations and can assist organizations in preventing expensive errors, as data-related mistakes cost U.S. businesses an estimated $3.1 trillion each year. Frequent audits offer a solid justification for guaranteeing that adherence measures are effectively implemented.
Stakeholder Engagement: Foster open communication with stakeholders, including regulatory bodies and ethics committees, to ensure transparency and collaboration. Engaging stakeholders effectively can lead to better adherence results and enhance trust in your organization. Establishing anonymous reporting channels can motivate employees to report regulatory issues without fear of retaliation, further enhancing your regulatory framework.
By applying these procedures, you will create a strong regulatory framework that facilitates continuous adherence to medical devices compliance, ultimately aiding the success of your healthcare projects.

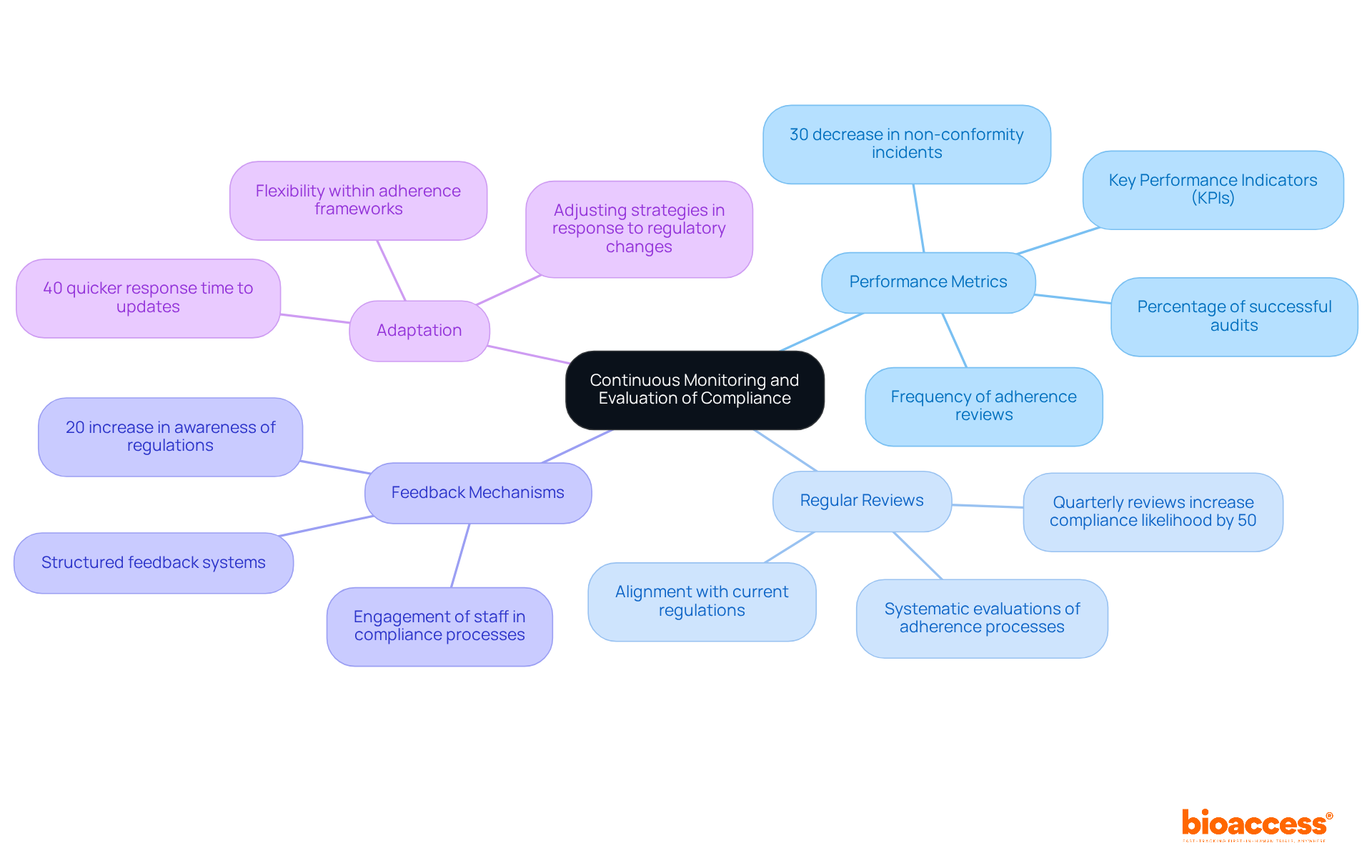
Establishing a robust system for ongoing monitoring and assessment of medical devices compliance is essential for maintaining regulatory standards in the medical device industry. This encompasses several critical components:
Performance Metrics: It is imperative to clearly define key performance indicators (KPIs) that effectively measure compliance. Metrics such as the frequency of adherence reviews and the percentage of successful audits are instrumental in pinpointing areas that require enhancement. Notably, organizations that conduct regular adherence assessments often report a 30% decrease in non-conformity incidents.
Regular Reviews: Systematic evaluations of adherence processes and procedures must be scheduled to ensure alignment with current regulations and best practices. Data indicates that companies conducting quarterly reviews are 50% more likely to achieve medical devices compliance compared to those with annual assessments.
Feedback Mechanisms: Implementing structured feedback systems is crucial for gathering insights from team members and stakeholders regarding adherence challenges and successes. Engaging staff in this process can yield a 20% increase in awareness of regulations and proactive issue reporting.
Adaptation: Organizations must be prepared to adjust their adherence strategies in response to regulatory changes or improvements in internal processes. Flexibility within adherence frameworks positions organizations to manage evolving regulations effectively, as evidenced by case studies that show a 40% quicker response time to updates.
By committing to continuous monitoring and evaluation, your efforts in medical devices compliance will remain effective and responsive to the dynamic regulatory landscape, ultimately safeguarding patient safety and enhancing product reliability.
Mastering medical device compliance is not merely a regulatory obligation; it is a critical endeavor that shapes the future of healthcare innovations. A comprehensive grasp of safety, efficacy, risk management, and quality management systems is essential for organizations aiming to thrive in this intricate landscape. By concentrating on these core principles, companies can adeptly navigate the regulatory complexities, ensuring their products not only meet but exceed the necessary standards for market success. Collaboration with specialized contract research organizations further streamlines this compliance journey, significantly enhancing the prospects for successful commercialization.
The key insights presented underscore the necessity of:
Each step—from conducting gap analyses and allocating resources to establishing standard operating procedures and engaging stakeholders—is vital for cultivating an environment of adherence and accountability. Furthermore, continuous monitoring and evaluation of compliance measures are imperative, allowing organizations to remain agile in response to evolving regulations while upholding high standards for patient safety.
Ultimately, the significance of medical device compliance transcends mere regulatory requirements; it directly influences patient outcomes and the overarching success of healthcare innovations. By prioritizing compliance and adopting a proactive stance, organizations can mitigate risks while contributing to a safer, more effective healthcare landscape. Taking decisive actions toward compliance today will lay the groundwork for a more reliable and trustworthy future in medical device technology.
What are the core principles of medical device compliance?
The core principles of medical device compliance include safety and efficacy, risk management, and quality management systems (QMS). These principles ensure that medical devices are safe for users and effective for their intended purposes.
How is safety and efficacy established in medical devices?
Safety and efficacy are established through rigorous testing and robust clinical evidence. An example is the FDA's endorsement of Boston Scientific’s WATCHMAN FLX™ product, which demonstrates effective solutions for stroke prevention in atrial fibrillation patients.
Why is risk management important in medical device compliance?
Risk management is crucial for identifying potential risks associated with medical devices. It involves systematic documentation and adherence to standards like ISO 14971, which guides manufacturers in assessing and mitigating risks throughout the product lifecycle.
What role do Quality Management Systems (QMS) play in medical device compliance?
QMS principles are essential for maintaining product quality throughout the development process. A well-implemented QMS ensures compliance with quality standards from design to post-market evaluation, improving clinical results and compliance assurance.
How can specialized contract research organizations (CROs) assist with medical device compliance?
Specialized CROs, such as bioaccess®, can enhance compliance by providing expertise in accelerating clinical trials, regulatory approval, clinical research site activation, and patient recruitment, helping manage the complexities of regulations.
What are the key regulatory frameworks for medical devices in the USA?
In the USA, the key regulatory framework is overseen by the FDA, particularly through 21 CFR Part 820, which outlines the Quality System Regulation (QSR) for medical devices.
What is the significance of the European Medical Device Regulation (EU MDR)?
The EU MDR is crucial for products marketed in Europe, imposing stringent safety and performance requirements. Manufacturers must also inform authorities of anticipated supply disruptions for specific products starting in early 2025.
What ISO standards are relevant to medical device compliance?
ISO standards, particularly ISO 13485, provide essential guidelines for quality management systems related to medical equipment. Adhering to these standards aids in obtaining approval and contributes to overall compliance and product safety.
Why is it important to investigate specific rules for medical devices?
Investigating the specific rules that pertain to your equipment category and market is vital for upholding standards and ensuring successful commercialization of medical devices.