


Phase 0 clinical trials are essential preliminary studies designed to gather critical pharmacokinetic and pharmacodynamic data from a select group of participants, without the expectation of therapeutic outcomes. These trials play a pivotal role in expediting drug development processes, allowing for the early identification of viable candidates. Moreover, they adhere to ethical and regulatory standards, which enhances the overall efficiency and success rate of subsequent clinical phases. The significance of these trials cannot be overstated, as they lay the groundwork for more advanced studies, ensuring that the journey from concept to market is both effective and responsible.
Phase 0 clinical trials, often underestimated in the drug development landscape, are crucial in shaping the future of innovative therapies. These exploratory studies present a unique opportunity for researchers to gather essential insights into pharmacokinetics and pharmacodynamics with minimal risk, ultimately facilitating the journey toward effective treatments.
As the field evolves, however, it is imperative to consider the challenges and ethical considerations that innovators must navigate to fully leverage the potential of Phase 0 trials.
Phase 0 in clinical trials, often referred to as exploratory studies or microdose investigations, represents the initial stage of human testing, typically involving fewer than 15 participants. These experiments primarily aim to gather foundational data on pharmacokinetics (PK) and pharmacodynamics (PD) of a substance, without the expectation of therapeutic benefit. This phase is crucial for understanding how the medication interacts within the human body, determining optimal dosing strategies, and refining target selection for subsequent development stages.
By utilizing subtherapeutic amounts, initial studies significantly expedite the medication development process, enabling researchers to make informed decisions about advancing to Stage I evaluations or halting further development. Recent advancements in microdosing techniques and regulatory flexibility have further enhanced the efficacy of these studies, establishing them as a vital component of modern drug development.
In 2025, the number of phase 0 in clinical trials conducted reflects a growing recognition of their importance, with numerous success stories emerging from these early-stage investigations, demonstrating their ability to streamline the pathway to effective therapies.
At bioaccess, we provide comprehensive clinical research management services, including:
Our expertise empowers startups to navigate the complex regulatory landscape, ensuring a smoother approval process and ultimately contributing to the success of their clinical studies.

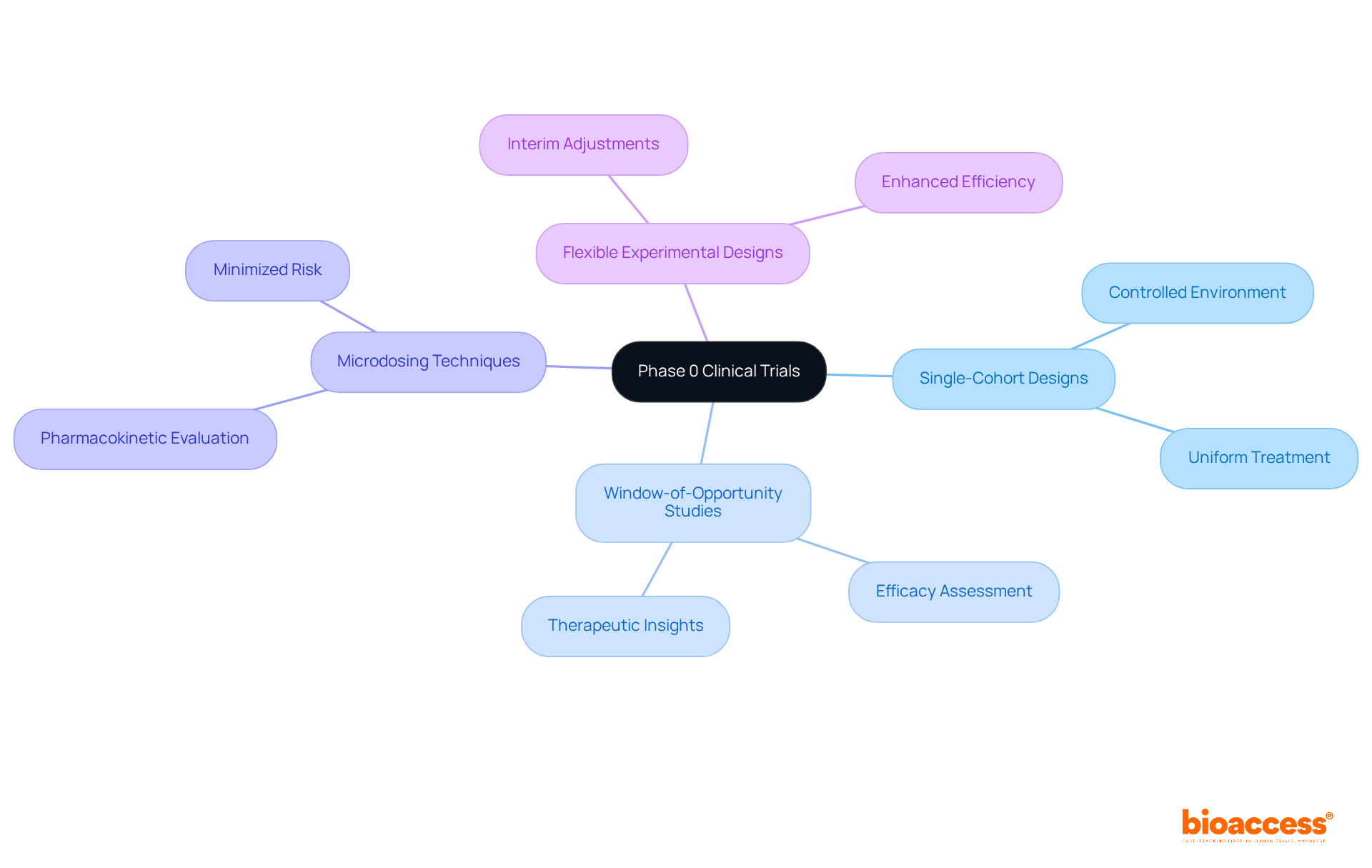
In phase 0 in clinical trials, diverse methodologies are employed, prominently featuring single-cohort designs where all participants receive the same treatment. This approach is particularly advantageous for evaluating the effects of substances within a controlled environment.
For example, window-of-opportunity studies allow researchers to assess a medication's efficacy in patients slated for standard treatments, thereby providing critical insights into its therapeutic potential.
Additionally, microdosing techniques, which involve administering very low doses, facilitate the evaluation of pharmacokinetic profiles while minimizing participant risk.
Furthermore, flexible experimental designs are increasingly utilized in early investigations, permitting adjustments based on interim outcomes. This adaptability enhances experimental efficiency and relevance, ensuring that the research remains aligned with its objectives and the evolving understanding of the drug's behavior in humans.
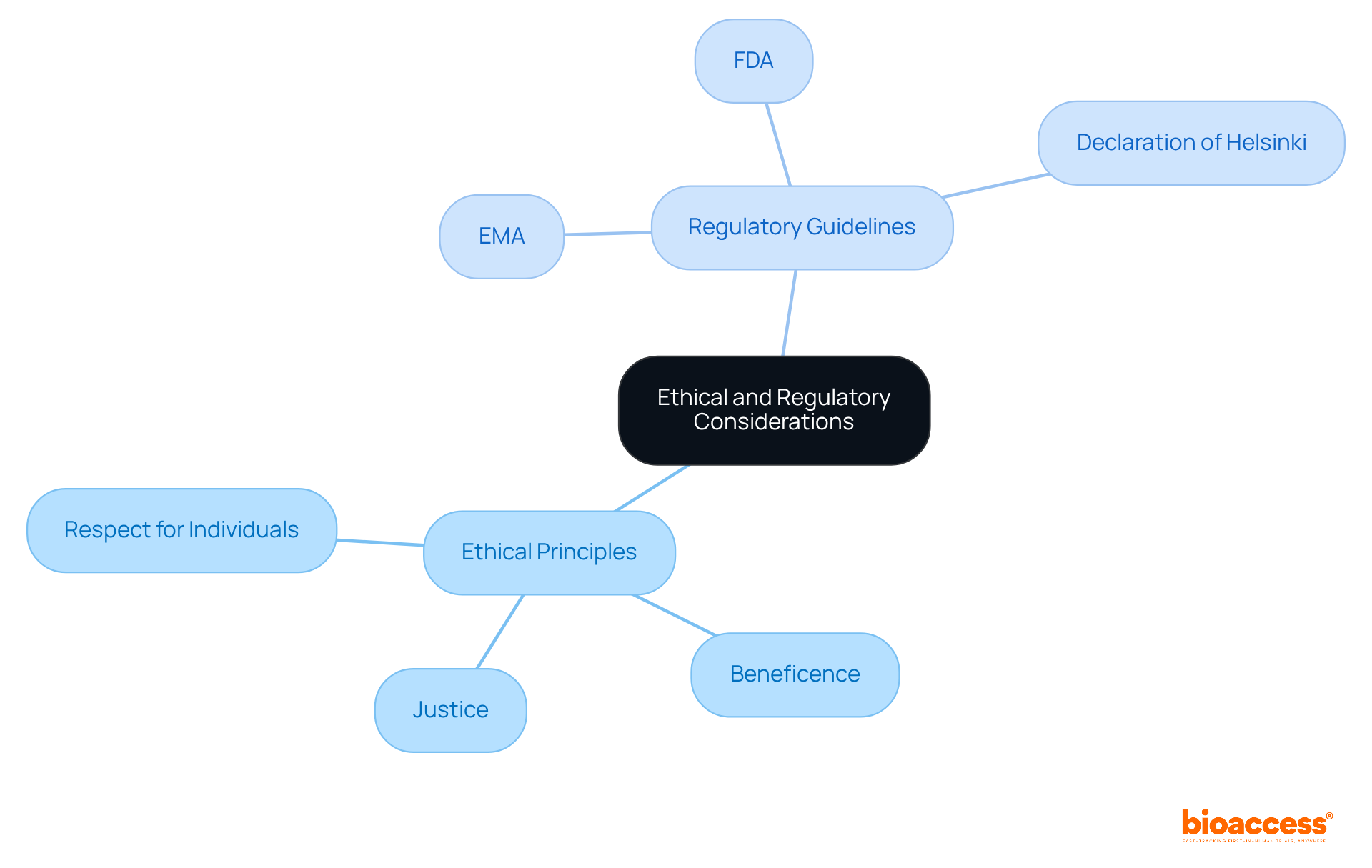
Navigating the ethical and regulatory environments of early-stage experiments is essential, as these assessments do not seek to offer therapeutic advantages. Informed consent is critical; participants must understand that they are part of a study designed to collect data rather than receive treatment. Regulatory agencies, such as the FDA and EMA, have established extensive guidelines for phase 0 in clinical trials, underscoring the importance of ethical supervision and compliance with the Declaration of Helsinki. Researchers bear the responsibility of upholding ethical principles such as:
Throughout the study process, as we look to 2025, these regulatory requirements continue to evolve, highlighting the significance of transparency and participant safety in exploratory studies.
Initial studies present significant advantages for creators within the Medtech, Biopharma, and Radiopharma sectors. By offering early insights into a medication's behavior in humans, these studies can substantially reduce both the duration and costs associated with medication development. They facilitate the early identification of non-viable candidates, enabling companies to direct their resources toward the most promising options.
Furthermore, the information quality in phase 0 in clinical trials enhances informed decision-making, leading to a more efficient transition to subsequent phases. This strategic approach not only accelerates development timelines but also increases the likelihood of successful market entry, establishing it as a crucial element of contemporary drug development strategies.
The expertise of bioaccess® in managing Early-Feasibility Studies, First-In-Human Studies, and other phases of clinical research—including site selection, compliance reviews, and project management—highlights the necessity of comprehensive clinical study management services.
Additionally, the influence of Medtech clinical studies on local economies, such as job creation and healthcare enhancement, underscores the wider benefits of these trials beyond the innovators themselves.

Phase 0 clinical trials represent a pivotal stage in the drug development process, serving as the first step in human testing to gather essential data on pharmacokinetics and pharmacodynamics. By employing innovative methodologies and adhering to strict ethical and regulatory guidelines, these exploratory studies enable researchers to make informed decisions about which compounds to advance, ultimately accelerating the path toward effective therapies.
Key insights throughout the article highlight the practical benefits of Phase 0 trials for innovators in the Medtech, Biopharma, and Radiopharma sectors. These trials not only reduce development timelines and costs but also enhance the quality of information available for decision-making. The emphasis on ethical considerations and compliance underscores the responsibility researchers hold in ensuring participant safety and transparency.
The significance of Phase 0 trials extends beyond individual companies; they play a crucial role in shaping the future of drug development and can positively impact local economies through job creation and healthcare advancements. As the landscape of clinical research continues to evolve, embracing the advantages of Phase 0 trials is essential for innovators aiming to navigate the complexities of the regulatory environment and achieve successful market entry.
What are Phase 0 clinical trials?
Phase 0 clinical trials, also known as exploratory studies or microdose investigations, are the initial stage of human testing that typically involves fewer than 15 participants. They aim to gather foundational data on pharmacokinetics (PK) and pharmacodynamics (PD) of a substance.
What are the objectives of Phase 0 clinical trials?
The primary objectives of Phase 0 trials are to understand how a medication interacts within the human body, determine optimal dosing strategies, and refine target selection for subsequent development stages, without the expectation of therapeutic benefit.
How do Phase 0 trials expedite the medication development process?
By utilizing subtherapeutic amounts, Phase 0 trials allow researchers to make informed decisions about whether to advance to Stage I evaluations or halt further development, thus significantly speeding up the medication development process.
What advancements have improved Phase 0 clinical trials?
Recent advancements in microdosing techniques and regulatory flexibility have enhanced the efficacy of Phase 0 studies, establishing them as a vital component of modern drug development.
What trends are observed in Phase 0 clinical trials as of 2025?
By 2025, there is a growing recognition of the importance of Phase 0 clinical trials, with numerous success stories emerging from these early-stage investigations, which demonstrate their ability to streamline the pathway to effective therapies.
What services does bioaccess provide in relation to clinical trials?
Bioaccess offers comprehensive clinical research management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting, to help startups navigate the regulatory landscape and ensure a smoother approval process.