


The article underscores the strategies essential for achieving success in Phase 2 clinical trials, which are pivotal for evaluating the effectiveness and safety of new interventions following initial safety confirmation. It highlights the critical importance of:
These elements are crucial for enhancing trial outcomes and facilitating the advancement of promising candidates to Phase 3 studies.
Phase 2 trials represent a pivotal point in the clinical research landscape, effectively bridging the gap between initial safety assessments and extensive efficacy evaluations. These studies not only determine the optimal dosage and evaluate treatment efficacy but also play a crucial role in shaping the future of drug development. With a staggering 60-70% of drugs failing to progress beyond this stage, the stakes are undeniably high.
What strategies can researchers implement to enhance the success of Phase 2 trials and ensure that only the most promising candidates advance to the next level?
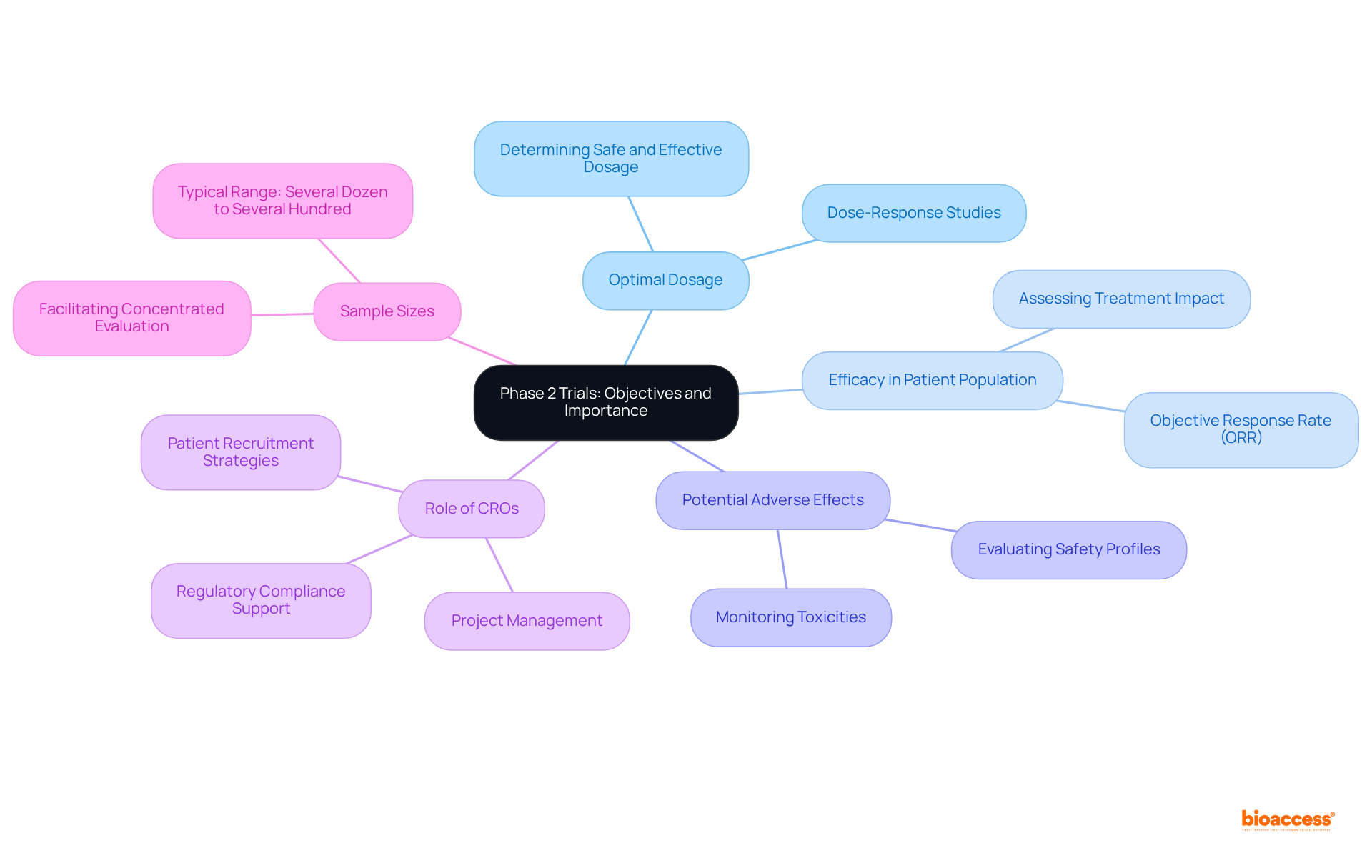
Phase 2 studies represent a pivotal phase in the clinical research process, primarily focused on evaluating the effectiveness and safety of an intervention following the initial safety confirmation in Stage 1 studies. The objectives of Stage 2 studies include:
Sample sizes for Stage 2 clinical studies typically range from several dozen to several hundred participants, facilitating a concentrated evaluation of the intervention's impact. This phase 2 is crucial as it delivers the first substantial evidence of the treatment's potential benefits, which in turn informs further development and investment decisions.
Moreover, the success rates of phase 2 studies significantly influence the progression of candidates to phase 3 studies, where larger cohorts are examined. Contract Research Organizations (CROs) enhance the efficiency and effectiveness of Stage 2 studies by providing project management, patient recruitment strategies, and regulatory compliance support, all of which contribute to the success of drug development.
Understanding the importance of phase 2 evaluations empowers stakeholders to appreciate their role in the comprehensive drug development process, ensuring that only the most promising candidates advance to stage 3 assessments.
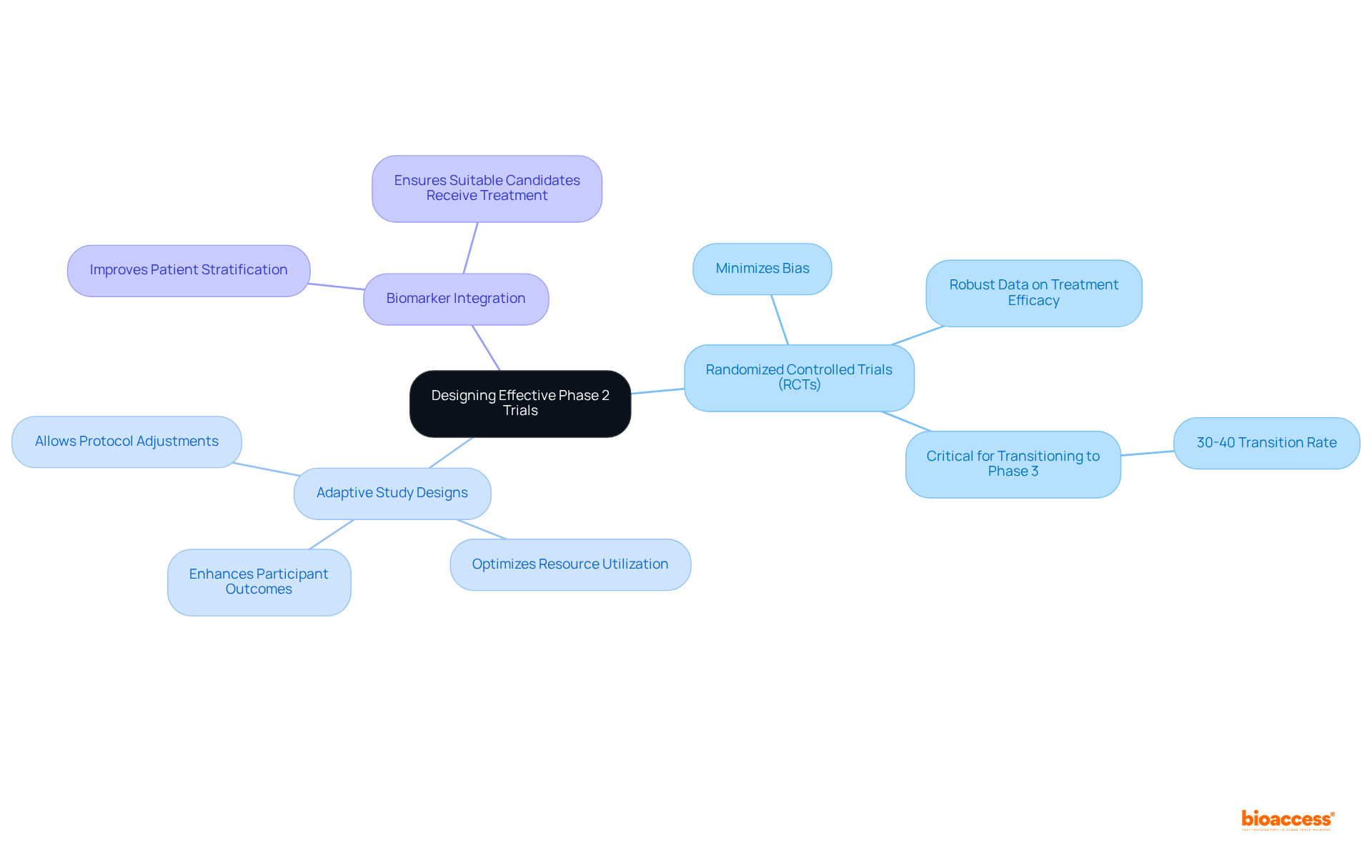
Creating successful Stage 2 studies necessitates a strategic choice of methodologies that align with the study's objectives. Randomized controlled trials (RCTs) stand as a cornerstone of this phase, minimizing bias and providing robust data on treatment efficacy. Statistics reveal that only approximately 30%-40% of drugs effectively transition from phase 2 to phase 3, underscoring the critical importance of well-structured studies.
Adaptive study designs are increasingly favored, allowing for adjustments to the study protocol based on interim results, which optimizes resource utilization and enhances participant outcomes. Furthermore, integrating biomarkers into study designs can significantly improve patient stratification, ensuring that the most suitable candidates receive the treatment.
By meticulously evaluating these approaches, researchers can develop studies in phase 2 that yield significant outcomes and facilitate informed decisions for subsequent stages.
Navigating regulatory compliance during phase 2 studies is paramount for conducting research ethically and legally. Researchers must be well-versed in the guidelines established by regulatory bodies such as the FDA and EMA, which detail requirements for study design, informed consent, and data reporting. Key aspects include:
By comprehensively understanding and following these regulatory requirements, researchers can significantly mitigate risks and enhance the credibility of their findings. This, in turn, contributes to the advancement of medical knowledge. Successful IND applications in phase 2 studies exemplify the effective integration of these guidelines, underscoring the importance of adherence in achieving favorable outcomes.
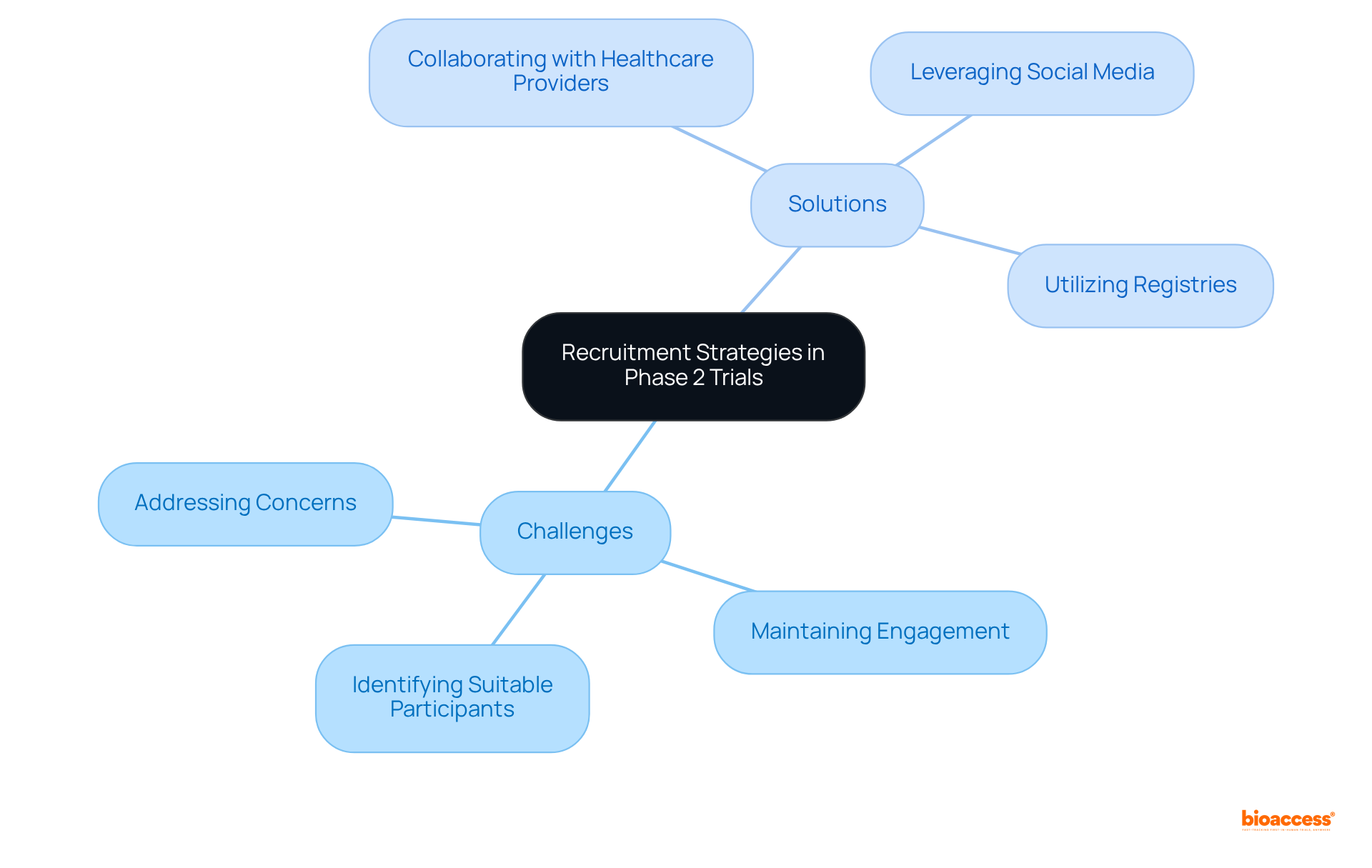
Implementing effective recruitment strategies is crucial for the success of studies in phase 2, as participant enrollment directly impacts both the schedule and outcomes. Common challenges include:
To tackle these challenges, researchers can employ targeted outreach strategies, such as:
Furthermore, providing clear details regarding the study's purpose, potential advantages, and risks can alleviate participant concerns. Notably, 80% of all medical studies fail to meet their participant enrollment targets, highlighting the widespread nature of these challenges. Additionally, 70% of individuals eligible for a clinical study live more than two hours from a research center, complicating participation logistics. By proactively addressing these recruitment challenges, researchers can improve enrollment rates and ensure that trials are completed efficiently.
As Marta Garcia Manrique, R&D Chief Patient Officer at Servier, states, 'The pharmaceutical industry must explore new recruitment procedures, specifically leveraging social networks and strengthening relationships with patient organizations.' This approach aligns with the success observed in studies facilitated by bioaccess®, which achieve enrollment 50% faster than traditional methods.
Phase 2 trials represent a critical juncture in clinical research, concentrating on the effectiveness and safety of new interventions following initial safety assessments. These studies are essential for determining optimal dosages, evaluating efficacy in targeted patient populations, and identifying potential adverse effects. The insights gained during this phase are instrumental in guiding further drug development and investment decisions, ultimately shaping the future landscape of medical treatments.
The article has explored key strategies for conducting successful Phase 2 trials, emphasizing the importance of:
Randomized controlled trials and adaptive study designs emerge as vital approaches for minimizing bias and optimizing outcomes. Furthermore, understanding and adhering to regulatory requirements is paramount for ethical and credible research, while overcoming recruitment challenges ensures that trials can be completed efficiently and effectively.
In conclusion, the success of Phase 2 trials is not only pivotal for advancing promising drug candidates but also for enhancing the overall integrity and efficiency of clinical research. Stakeholders are encouraged to adopt best practices in trial design, prioritize regulatory adherence, and innovate in recruitment strategies. By doing so, the clinical research community can significantly improve the likelihood of successful outcomes, ultimately benefiting patients and the healthcare system at large.
What are Phase 2 trials?
Phase 2 trials are clinical studies that evaluate the effectiveness and safety of an intervention after initial safety confirmation in Stage 1 studies.
What are the main objectives of Phase 2 studies?
The main objectives of Phase 2 studies include determining the optimal dosage, assessing the intervention's efficacy within a specific patient population, and identifying any potential adverse effects.
What is the typical sample size for Phase 2 clinical studies?
Sample sizes for Phase 2 clinical studies typically range from several dozen to several hundred participants.
Why are Phase 2 trials considered crucial in the clinical research process?
Phase 2 trials are crucial because they provide the first substantial evidence of a treatment's potential benefits, which informs further development and investment decisions.
How do Phase 2 study success rates affect drug development?
The success rates of Phase 2 studies significantly influence whether candidates progress to Phase 3 studies, where larger cohorts are examined.
What role do Contract Research Organizations (CROs) play in Phase 2 studies?
CROs enhance the efficiency and effectiveness of Phase 2 studies by providing project management, patient recruitment strategies, and regulatory compliance support.
Why is it important to understand the significance of Phase 2 evaluations?
Understanding the importance of Phase 2 evaluations helps stakeholders appreciate their role in the drug development process, ensuring that only the most promising candidates advance to Stage 3 assessments.