


The article underscores the critical importance and implementation of Phase I studies in clinical research, highlighting their essential role in evaluating drug safety, pharmacokinetics, and appropriate dosing. It elaborates on the objectives of these studies, detailing the subtypes:
It also covers the regulatory compliance requirements and recruitment strategies. Furthermore, it emphasizes the significance of data analysis in steering future clinical development, reinforcing the necessity of these studies in advancing medical research.
Phase I studies serve as the critical foundation of clinical research, where the safety and efficacy of new drugs are first tested on human subjects. These initial trials not only determine the maximum tolerated dose but also provide essential pharmacokinetic data that can shape future therapeutic strategies. However, with nearly 80% of clinical studies facing delays due to recruitment challenges, Clinical Research Directors must navigate the complexities of Phase I trials effectively to ensure successful outcomes. This article delves into the objectives, subtypes, regulatory considerations, and data analysis of Phase I studies, equipping professionals with key insights to enhance their clinical research endeavors.
The phase I study examinations represent the crucial initial stage of clinical research involving human subjects, typically conducted with a small cohort of 20 to 100 healthy volunteers. The primary objectives of these studies encompass:
The significance of the phase I study in drug development is profound. These phase I studies provide the preliminary information necessary to validate further clinical advancement, ensuring that subsequent studies are conducted securely and efficiently. As highlighted by clinical research specialists, "The safety evaluation in Stage I trials is crucial; it not only safeguards participants but also enhances the credibility of the overall clinical trial process." In 2025, the role of Stage I research continues to evolve, underscoring its importance in the medication development landscape.

Phase I studies are fundamentally categorized into two critical subtypes: Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD), with each serving a pivotal role in the early phases of therapeutic development.
Single Ascending Dose (SAD): This design entails administering a single dose of the investigational substance to participants, with subsequent cohorts receiving progressively higher doses. This method is particularly advantageous for identifying the maximum tolerated dose (MTD) and establishing the initial safety profile of the drug, free from the complications associated with repeated dosing. The simplicity of SAD facilitates effective data collection on safety and tolerability, making it a preferred choice in early-stage studies. Notably, 48.39% of dose-escalation trials did not achieve their MTD during the trial, highlighting the challenges faced in these investigations.
Multiple Ascending Dose (MAD): Conversely, MAD trials require participants to receive multiple doses of the medication over a defined duration. This design enables researchers to evaluate the pharmacokinetics and safety of the medication over time, offering a more comprehensive understanding of its effects. MAD studies are essential for assessing how the substance behaves in the body with repeated exposure, which is crucial for determining appropriate dosing schedules for subsequent phases.
Both SAD and MAD designs are indispensable for ascertaining a medication's safety and effectiveness, providing vital information that guides the transition to the phase I study. Current trends indicate a growing preference for these designs due to their ability to streamline the development process and enhance patient safety. As articulated by Paul B Tchounwou, 'Stage 1 clinical trials signify an essential stage of medication development because new candidate therapeutic agents are evaluated for the first time on humans.' This structured approach not only enhances the efficiency of phase I studies but also promotes informed decision-making regarding subsequent development stages. Real-world examples, such as the incorporation of a Dose Expansion Stage in Stage I Oncology Trials, illustrate the practical implications of these designs in optimizing drug development.

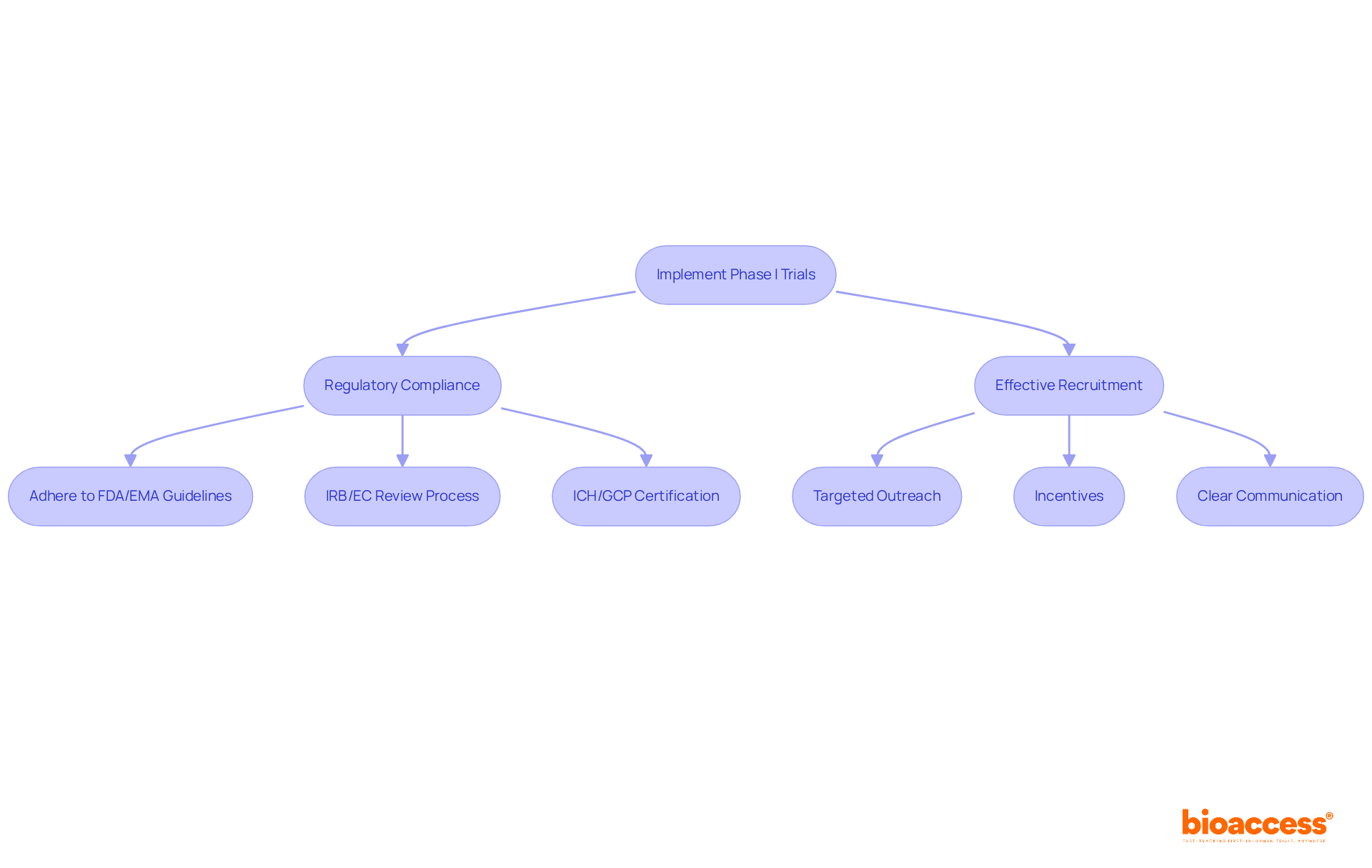
Implementing Phase I trials necessitates several key considerations:
Effective recruitment is essential for the success of a phase I study. Strategies may include:
By concentrating on these aspects, Clinical Research Directors can significantly enhance the likelihood of successful phase I study execution, leveraging Colombia's competitive strengths in cost effectiveness, regulatory agility, and patient enrollment.
Analyzing the results of Phase I trials involves several critical steps:
Data Interpretation: Researchers must meticulously evaluate safety data, pharmacokinetic profiles, and any observed adverse effects. This thorough examination is crucial for assessing whether a medication is safe for advancement to Stage II studies. A favorable safety profile coupled with optimal pharmacokinetics can lead to the green light for larger-scale studies, while significant safety concerns may necessitate halting further development. Additionally, the feasibility and selection of research sites and principal investigators (PIs) play a crucial role in ensuring that the data collected is robust and reliable.
Clinical Implications: Understanding the implications of the data is paramount. The results of the phase I study can significantly impact following research directions. If a medication demonstrates potential, it may lead to broader testing, improving the chances for successful market introduction. Conversely, adverse findings can redirect focus to alternative therapeutic avenues or necessitate modifications to the medication formulation. Comprehensive clinical study management services, including compliance reviews, study setup, import permits, and nationalization of investigational devices, become vital in navigating the complexities of drug development.
Reporting Findings: Results should be compiled into a comprehensive report that includes actionable recommendations for future studies. This ensures transparency and facilitates informed decision-making among stakeholders, including regulatory bodies and investors. Current trends emphasize the importance of clear and concise reporting, which not only aids in regulatory submissions but also enhances the credibility of the research. Effective project management and monitoring throughout the trial process are essential to ensure that all findings are accurately documented and communicated.
By effectively analyzing the results of the phase I study, Clinical Research Directors can make strategic decisions that significantly impact the trajectory of drug development, ultimately leading to more efficient pathways for bringing innovative therapies to market.

Phase I studies are integral to the clinical research landscape, serving as the preliminary phase where new drugs are first evaluated for safety and pharmacokinetics in human subjects. These trials establish essential safety profiles and dosing strategies critical for the success of subsequent phases in drug development.
Throughout this article, key aspects of Phase I studies were explored, including their objectives such as safety assessment, pharmacokinetics, and dose ranging. The distinctions between Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) designs were highlighted, emphasizing their roles in ensuring participant safety and gathering vital data. Furthermore, the discussion on regulatory compliance and effective recruitment strategies underscored the importance of adhering to guidelines and engaging diverse participant pools to enhance study outcomes.
The insights presented reinforce the significance of Phase I studies in the broader context of drug development. As the foundation upon which future research is built, these trials not only safeguard participant health but also streamline the pathway to innovative therapies. Clinical Research Directors are encouraged to leverage the strategies discussed to optimize their Phase I study processes, ultimately contributing to the advancement of safe and effective medical treatments.
What are Phase I studies in clinical research?
Phase I studies are the initial stage of clinical research involving human subjects, typically conducted with a small group of 20 to 100 healthy volunteers.
What are the primary objectives of Phase I studies?
The primary objectives of Phase I studies include safety assessment, pharmacokinetics, and dose ranging.
Why is safety assessment important in Phase I studies?
Safety assessment is crucial for determining the safety profile of a new drug or treatment, identifying adverse effects, and establishing a maximum tolerated dose (MTD), which protects participants in subsequent phases of clinical research.
What role does pharmacokinetics play in Phase I studies?
Pharmacokinetics involves understanding how the drug is absorbed, distributed, metabolized, and excreted in the body, which informs dosing strategies and enhances therapeutic efficacy in later stages.
What is dose ranging and why is it significant?
Dose ranging establishes the appropriate dosage for further studies, ensuring patient safety and mitigating risks associated with overdosing or underdosing in subsequent phases.
How do Phase I studies impact the overall clinical trial process?
Phase I studies provide preliminary information necessary for validating further clinical advancement, ensuring that subsequent studies are conducted securely and efficiently, thereby enhancing the credibility of the clinical trial process.
What challenges do Phase I studies face?
One significant challenge is recruitment, as 80% of clinical studies face delays or termination due to difficulties in recruiting participants.
How is the role of Phase I research expected to evolve in the future?
The role of Phase I research continues to evolve, underscoring its importance in the medication development landscape as of 2025.