

The article emphasizes the mastery of the probability of success (PoS) in clinical research, quantifying the likelihood that a study will meet its primary endpoints and yield favorable results. It elaborates on various types of PoS, including:
These types highlight their critical role in enhancing decision-making, optimizing resource allocation, and refining study design to improve drug development outcomes.
The landscape of clinical research is increasingly defined by the probability of success (PoS), a crucial metric that empowers stakeholders to gauge the likelihood of achieving favorable outcomes in their studies. Understanding PoS not only informs the feasibility of research but also enhances resource allocation and risk management, ultimately shaping the future of drug development. However, with varying success rates across different phases and therapeutic areas, researchers must consider:
The probability of success (PoS) in clinical research quantifies the likelihood that a clinical study will meet its primary endpoints and produce favorable results. This metric is vital for stakeholders, enabling them to evaluate the feasibility and the probability of success of a study before its initiation. PoS is influenced by various elements, including study design, patient population characteristics, and the specific intervention being evaluated.
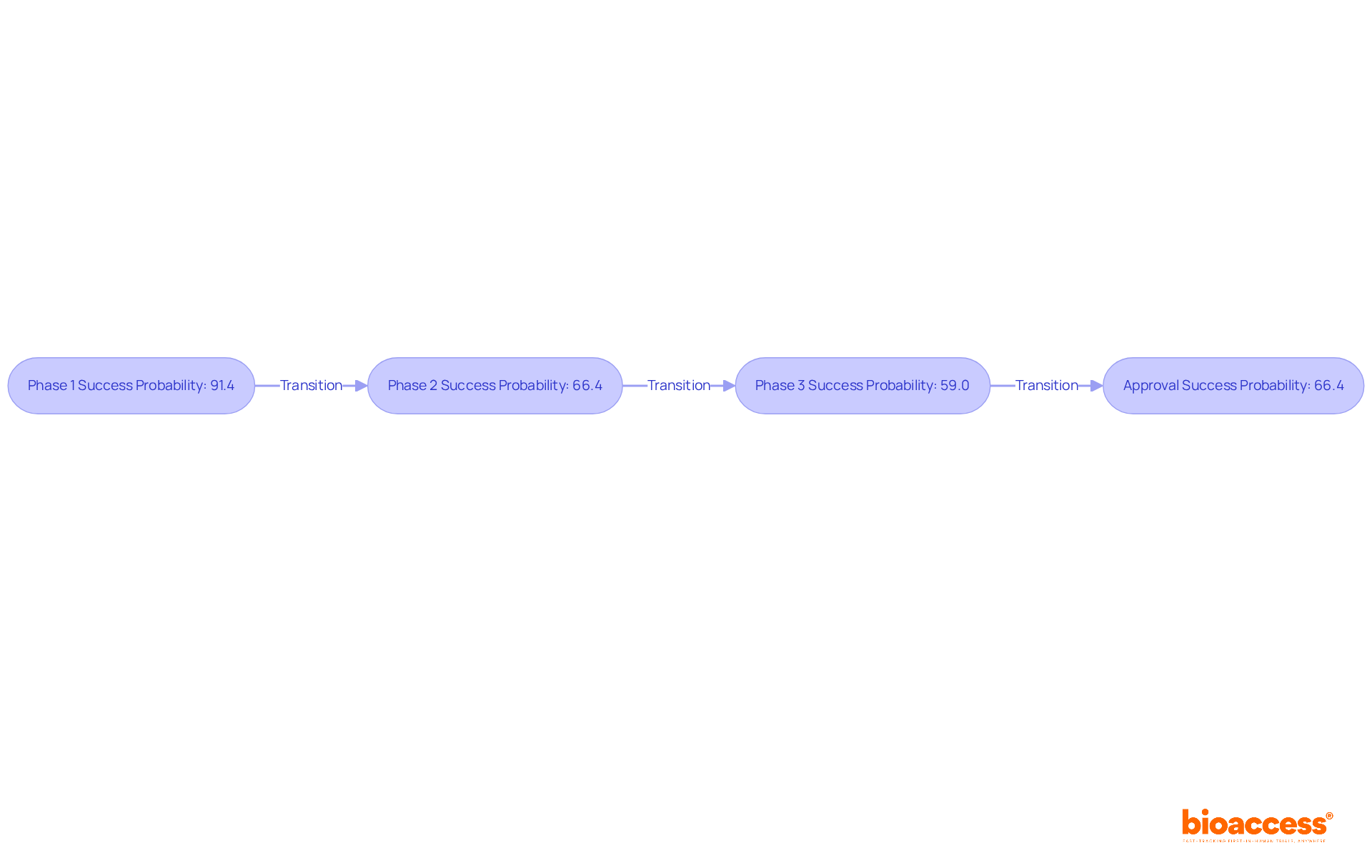
Recent studies reveal that the overall PoS for drug development programs has varied significantly over the years. For instance, the likelihood of success from Phase 1 to Approval stands at 66.4%, while completion rates for different phases are notably high, with Phase 1 studies achieving a completion rate of 91.4%. In contrast, oncology studies present a more challenging landscape, with a median duration of 13.1 years and a lower overall PoS of merely 3.4%.
To calculate PoS, researchers typically rely on historical data from similar experiments, statistical modeling, and expert judgment. For example, if historical data indicates a 70% success rate for analogous experiments, this figure serves as a baseline for estimating the PoS of a new study. This approach is essential for informed decision-making regarding resource allocation, study design, and risk management in medical research.
Experts emphasize the importance of understanding the probability of success, as it not only aids in assessing the feasibility of research studies but also helps to navigate the complexities of drug development. By leveraging insights from previous experiments and current trends, stakeholders can significantly enhance their studies' chances for success.
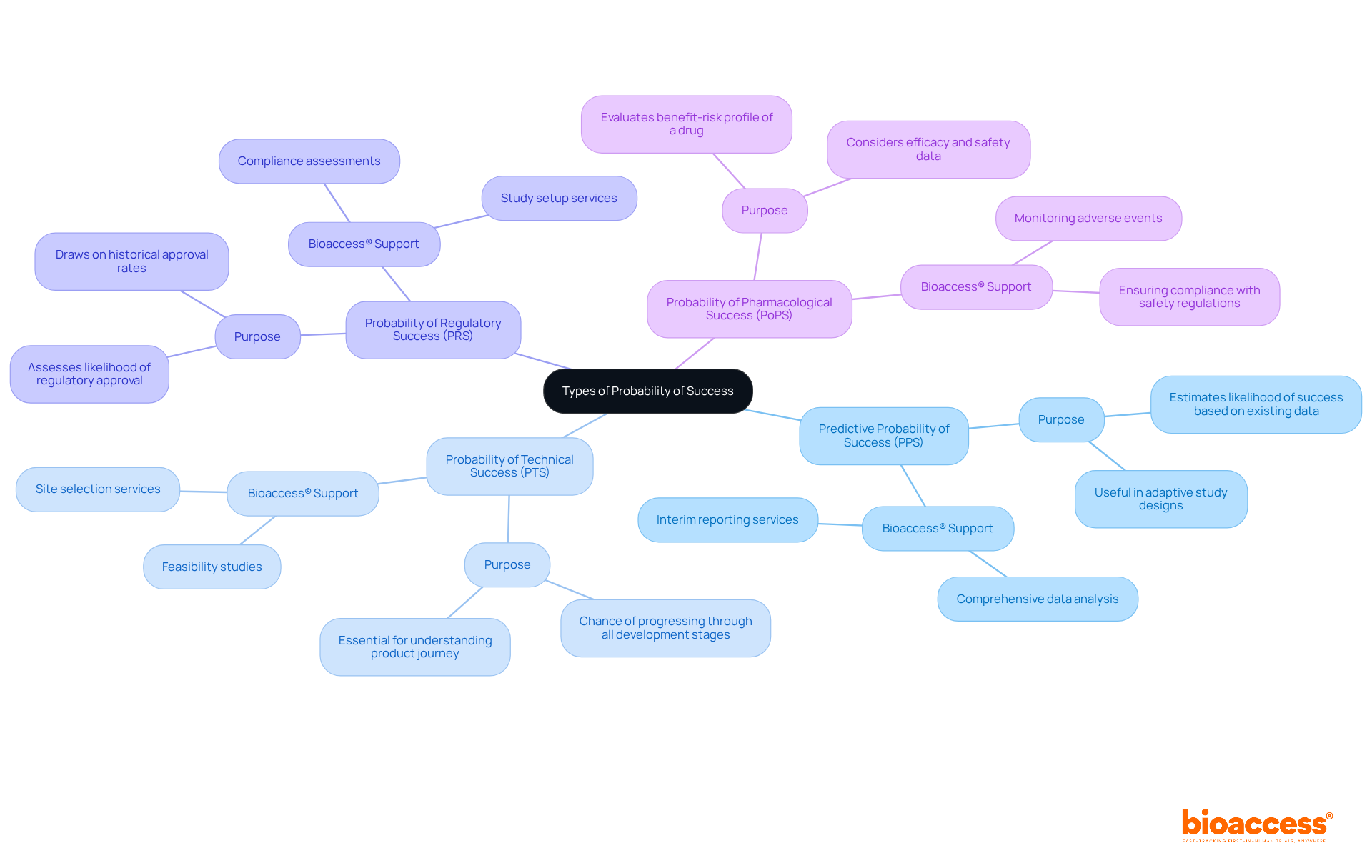
In clinical research, various types of probability of success serve distinct purposes, each critical for informed decision-making. These include:
Predictive Probability of Success (PPS): This type estimates the likelihood of success based on existing data, particularly useful in adaptive study designs. PPS enables researchers to make real-time modifications to study protocols based on interim results, thereby improving the overall efficiency of the research. Bioaccess® supports this by providing comprehensive data analysis and interim reporting services.
Probability of Technical Success (PTS): PTS emphasizes the chance that a drug or device will effectively progress through all stages of development, from preclinical studies to market approval. This metric is essential for understanding the comprehensive journey of a product. Bioaccess® aids in this area by providing feasibility studies and site selection services to ensure optimal testing conditions.
Probability of Regulatory Success (PRS): PRS assesses the likelihood of a product receiving regulatory approval, drawing on historical approval rates and the specific characteristics of the product. This evaluation is crucial for anticipating potential hurdles in the regulatory landscape. Given the complex regulatory requirements that early-stage studies encounter, Bioaccess® accelerates approval processes for startups by offering essential services such as compliance assessments and study setup, ensuring that products meet the necessary standards for regulatory success.
Probability of Pharmacological Success (PoPS): PoPS evaluates the chances of achieving a favorable benefit-risk profile for a drug, considering both efficacy and safety data. This metric is vital for ensuring that the therapeutic benefits outweigh potential risks. Bioaccess® contributes by monitoring adverse events and ensuring compliance with safety regulations.
A comprehensive grasp of these PoS types enables researchers and sponsors to make strategic choices concerning study structure, resource distribution, and to enhance the probability of success in risk management. As Uma Arumugam, MD, observed, the probability of success in drug development is greater than previously assessed, highlighting the significance of these metrics in managing the intricacies of medical studies. Furthermore, advancements in study design methodologies, such as adaptive designs and master protocols, have enhanced the efficiency of clinical studies, making it essential for stakeholders to leverage these insights for successful outcomes.
Designing pilot trials with a focus on Probability of Success (PoS) involves several critical steps:
Define Objectives: Clearly articulate the primary and secondary goals of the pilot experiment. This clarity aids in determining the endpoints to be measured and the criteria for success.
Select Appropriate Metrics: Choose metrics that align with the defined objectives. For example, if the objective is to evaluate safety, metrics should focus on adverse events and tolerability, ensuring relevance to the study's aims.
Estimate Sample Size: Utilize historical data and PoS calculations to determine the appropriate sample size for the pilot study. For instance, achieving 80% power typically requires sample sizes of 393, 100, and 64 for effect sizes of 0.20, 0.40, and 0.50, respectively. A well-calculated sample size enhances the reliability of the results and minimizes the risk of Type I and Type II errors.
Utilize Adaptive Approaches: Consider using adaptive study frameworks that permit adjustments based on interim findings. Such designs can significantly improve the PoS by enabling real-time adjustments to the experimental protocol, thus enhancing the study's responsiveness to emerging data.
Conduct Feasibility Evaluations: Before initiating the pilot study, carry out comprehensive feasibility evaluations to confirm the research can be implemented as intended. This includes evaluating site capabilities, patient recruitment strategies, and regulatory considerations, which are essential for identifying potential challenges early on. Utilizing bioaccess®'s knowledge in study setup, compliance evaluations, and project management can greatly improve this process.
By incorporating PoS into the framework of pilot studies, researchers can greatly improve the probability of success in larger investigations and optimize resource distribution, ultimately resulting in more effective research outcomes.

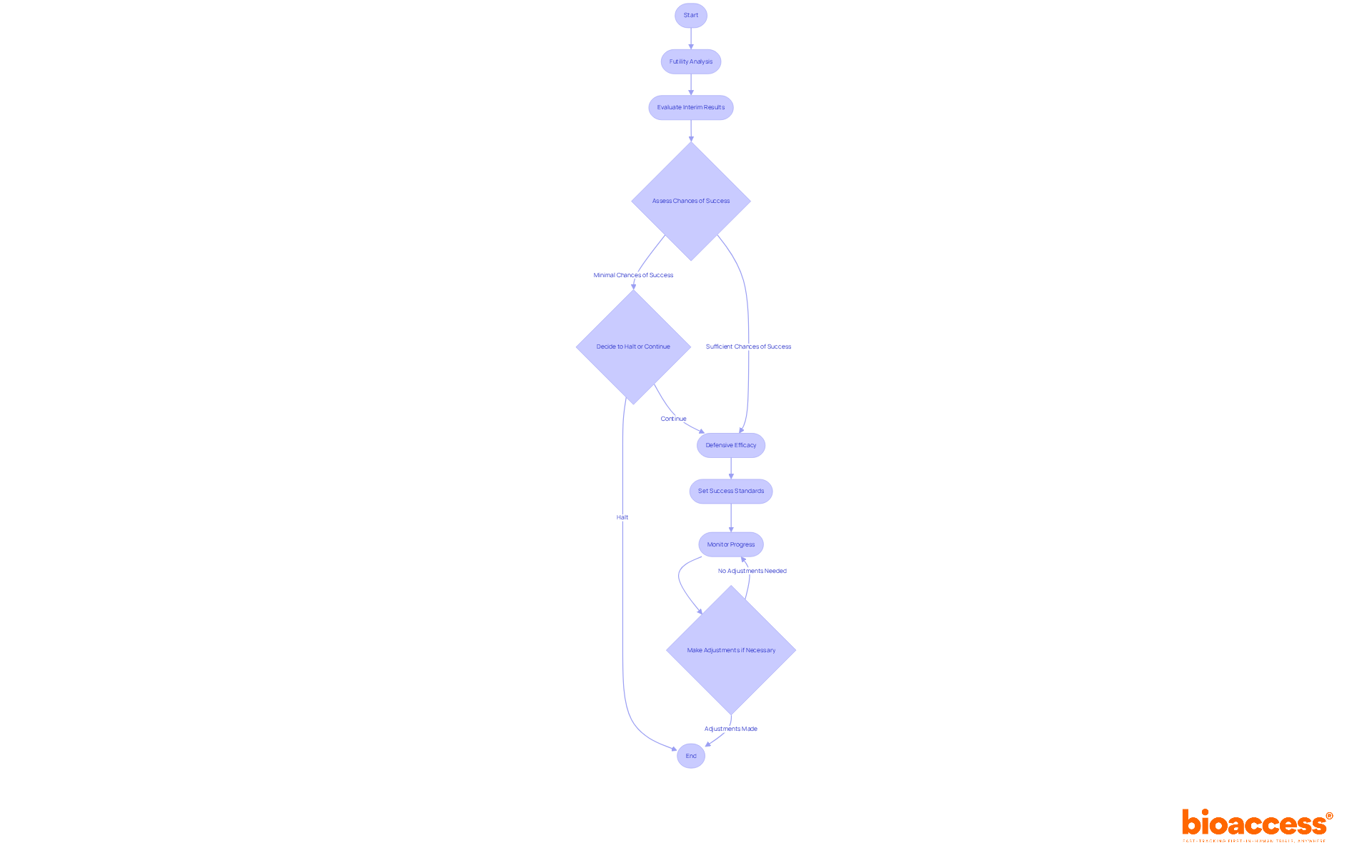
Implementing interim designs in clinical studies is crucial, particularly through the concepts of futility and defensive efficacy.
Futility Analysis is a critical evaluation performed to ascertain whether a study is unlikely to meet its objectives based on interim results. Should the analysis reveal minimal chances of success, it may be prudent to halt the experiment prematurely. This approach not only preserves valuable resources but also protects participants from ineffective treatments. For example, if interim data indicate a lack of substantial treatment effect, a futility analysis can decisively guide the decision to discontinue the study.
Defensive Efficacy emphasizes the necessity of ensuring that the experiment remains aligned with its objectives while mitigating risks. This strategy involves setting predetermined standards for success and diligently monitoring the study's progress against these benchmarks. If preliminary outcomes suggest that the treatment is not performing as expected, adjustments to the study structure or patient population can be made to enhance the likelihood of achieving favorable results.
By employing these interim designs, researchers are empowered to make informed decisions that optimize the probability of success while upholding ethical standards throughout the trial process.
Understanding the probability of success (PoS) in clinical research is essential for stakeholders aiming to navigate the complexities of drug development effectively. This article has explored the various dimensions of PoS, emphasizing its critical role in assessing the feasibility of studies and guiding decision-making processes. By recognizing the significance of PoS, researchers and sponsors can enhance their strategies, ultimately improving the chances of successful outcomes in clinical trials.
Key insights shared include the different types of PoS:
All serving unique functions in the research landscape. Furthermore, the article discussed the importance of designing pilot trials with PoS in mind, utilizing adaptive approaches, and implementing interim designs such as futility and defensive efficacy analyses to optimize study performance and resource allocation.
As the landscape of clinical research continues to evolve, embracing the principles of probability of success will be paramount. Stakeholders are encouraged to leverage these insights and methodologies to improve their projects and contribute to advancing medical science as a whole. By prioritizing PoS, the potential for more effective and efficient clinical trials becomes a tangible reality, paving the way for innovations that can benefit patients and healthcare systems alike.
What is the probability of success (PoS) in clinical research?
The probability of success (PoS) in clinical research quantifies the likelihood that a clinical study will meet its primary endpoints and produce favorable results.
Why is PoS important for stakeholders?
PoS is vital for stakeholders as it enables them to evaluate the feasibility and the probability of success of a study before its initiation.
What factors influence the probability of success in clinical research?
PoS is influenced by various elements, including study design, patient population characteristics, and the specific intervention being evaluated.
What are the overall PoS rates for drug development programs?
The overall PoS for drug development programs has varied, with the likelihood of success from Phase 1 to Approval at 66.4%.
What are the completion rates for different phases of clinical studies?
Completion rates are notably high, with Phase 1 studies achieving a completion rate of 91.4%. However, oncology studies have a lower overall PoS of 3.4% and a median duration of 13.1 years.
How is PoS calculated in clinical research?
Researchers typically calculate PoS using historical data from similar experiments, statistical modeling, and expert judgment. For instance, if historical data indicates a 70% success rate for analogous experiments, this serves as a baseline for estimating the PoS of a new study.
What is the significance of understanding PoS in drug development?
Understanding PoS aids in assessing the feasibility of research studies and helps navigate the complexities of drug development, enabling better decision-making regarding resource allocation, study design, and risk management.