


Mastering project management in clinical trials is essential for success, as it involves navigating unique challenges such as:
Effective strategies, including comprehensive planning and stakeholder engagement, significantly enhance project outcomes. This is evidenced by improved recruitment rates and adherence to regulatory standards achieved through expert management and collaboration. By addressing these challenges head-on, clinical trial managers can ensure a streamlined process that meets both regulatory requirements and patient needs.
Navigating the intricate landscape of clinical trials presents a unique set of challenges that can significantly impact outcomes. From stringent regulatory compliance to the complexities of patient recruitment, the stakes are high, and the margin for error is slim. This article delves into effective strategies for mastering project management in clinical trials, offering insights that can lead to improved efficiency and success rates. Organizations must consider how to transform these challenges into opportunities for advancement, ensuring that their trials not only meet regulatory standards but also achieve their objectives.
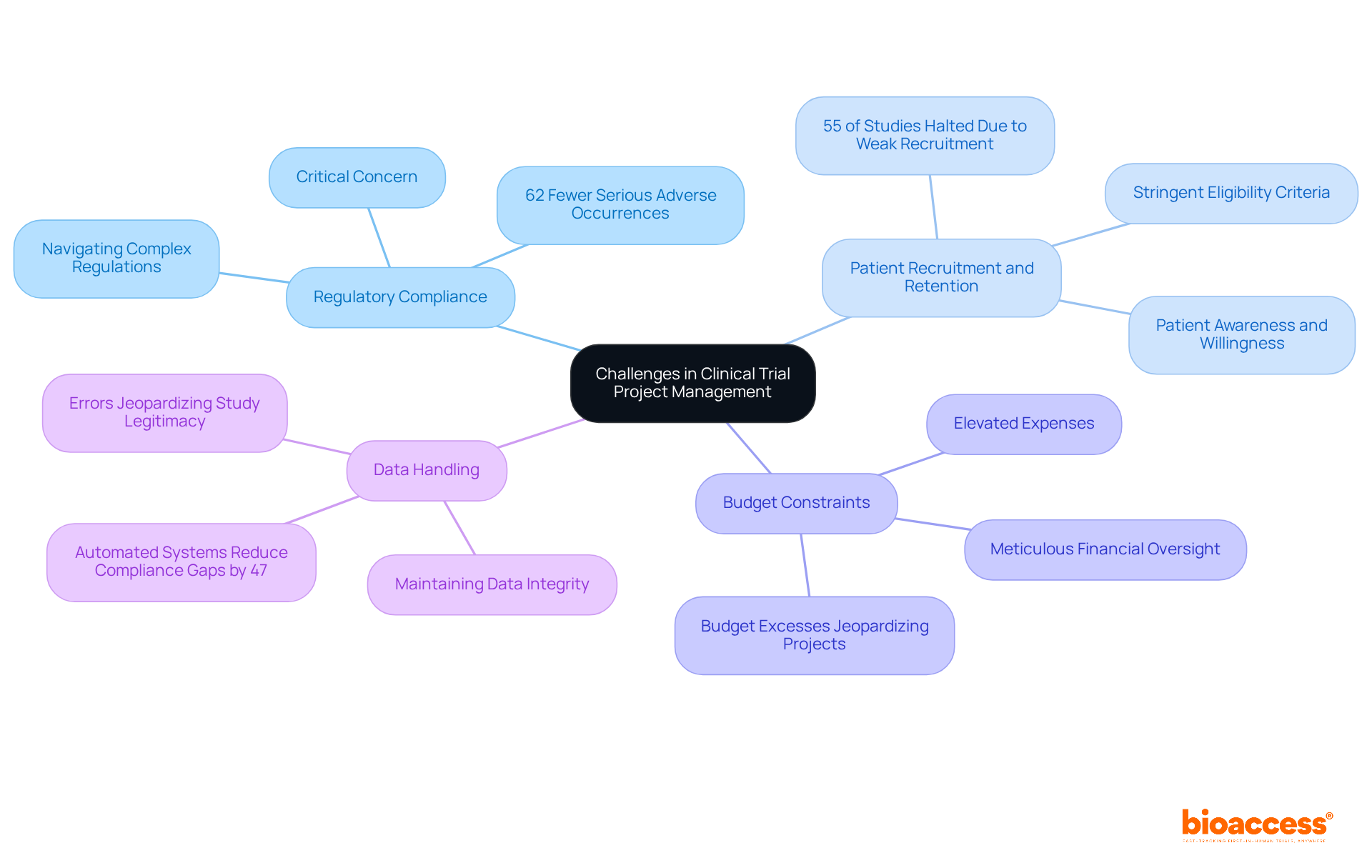
The distinct challenges of project management in clinical trials can significantly hinder progress and impact outcomes. Regulatory compliance stands out as a critical concern. Navigating the intricate web of regulations across various regions can be overwhelming; each jurisdiction has its own requirements, and non-compliance can lead to expensive delays or even the cancellation of studies. Companies with developed compliance abilities face 62% fewer serious adverse occurrences in research studies, underscoring the significance of adhering to regulatory standards.
In this context, bioaccess™ offers extensive project management in clinical trials services for studies, which include:
Their expertise guarantees that proceedings meet local regulations and standards, facilitating smoother operations. Additionally, bioaccess offers thorough review and feedback on study documents, which is essential for compliance with country-specific requirements.
Patient recruitment and retention also pose significant hurdles. Inadequate enrollment is the primary reason for study terminations, with research showing that 55% of investigations are halted due to weak recruitment. Factors such as stringent eligibility criteria, patient awareness, and willingness to participate can adversely affect recruitment rates. Notably, GlobalCare Clinical Studies collaborated with bioaccess™ to enhance its research ambulatory services in Colombia, achieving over a 50% decrease in recruitment time and a retention rate surpassing 95%. This collaboration illustrates how efficient project management in clinical trials can yield enhanced results.
Budget constraints further complicate the landscape of clinical studies. The elevated expenses linked to conducting experiments necessitate meticulous financial oversight; budget excesses can jeopardize the entire project. Effective financial management is essential in project management in clinical trials to ensure quality is maintained without exceeding allocated resources.
Data handling is another critical area where challenges arise. Maintaining data integrity and security is crucial, as ineffective data oversight can result in errors that jeopardize the legitimacy of the study. Automated systems for regulatory tracking have been shown to reduce compliance gaps by 47%, highlighting the role of technology in enhancing data management practices.
By identifying these challenges early in project management in clinical trials, managers can develop proactive strategies to address them efficiently, which ultimately leads to more successful studies. The expertise of professionals such as Ana Criado and Katherine Ruiz further bolsters the credibility of these strategies, ensuring that research studies are administered with the utmost standards of regulatory compliance and operational efficiency.
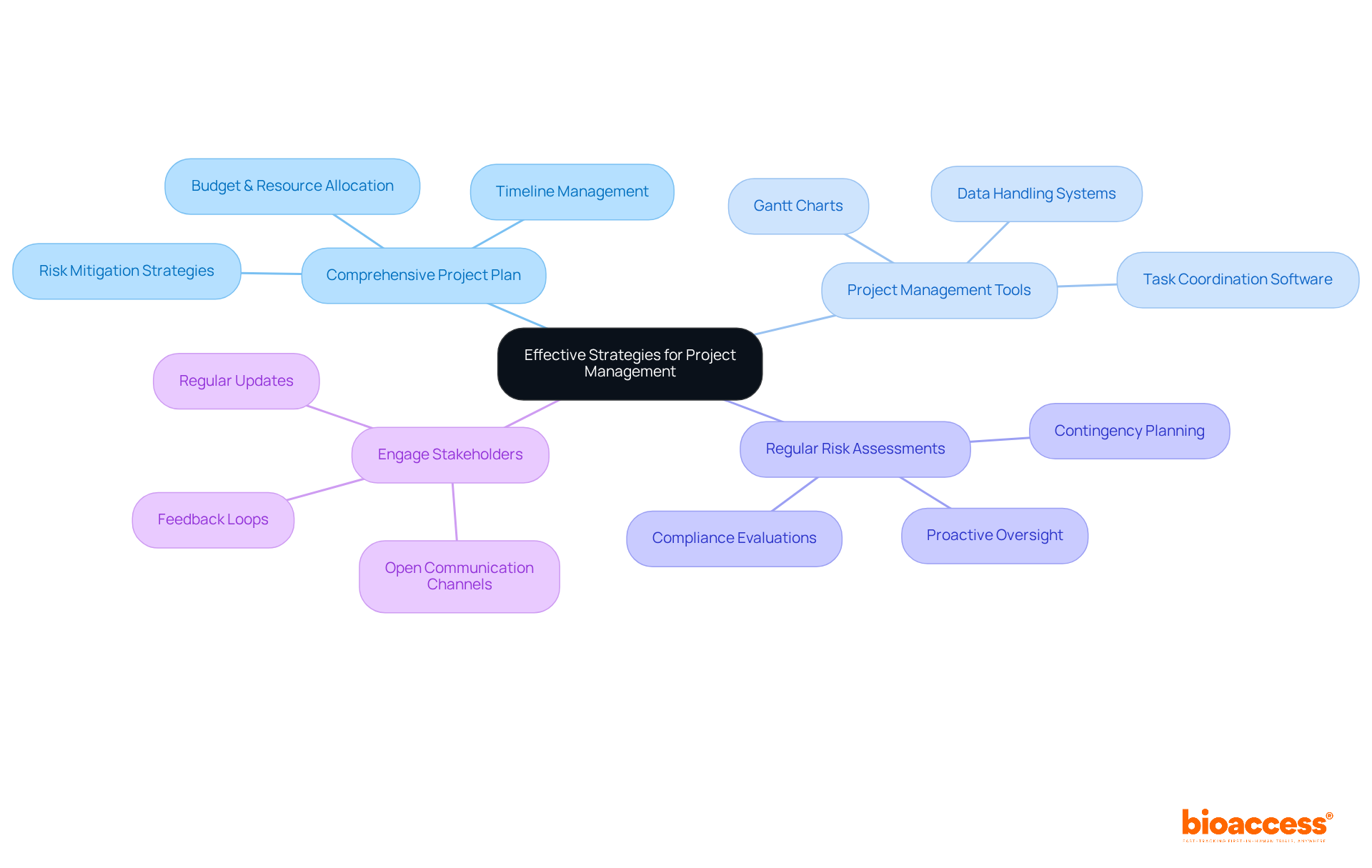
To navigate the complexities of clinical trial project management successfully, consider the following strategies:
Develop a Comprehensive Project Plan: A detailed project plan should outline all phases of the trial, including timelines, budgets, and resource allocation. This plan serves as a guide and assists in maintaining the initiative on course. Notably, the projected expense of introducing a new medication to the market is US$ 2.3 billion, underscoring the financial risks involved and the necessity for efficient oversight to regulate costs. Bioaccess provides extensive clinical study coordination services, including feasibility assessments and site selection, which can significantly simplify this process.
Utilize Project Management Tools: Leverage technology to streamline processes. Tools such as Gantt charts, task coordination software, and data handling systems can enhance efficiency and communication among team members. Efficient data handling systems are essential for ensuring the precision and safety of research data, which is crucial for upholding research integrity. With Bioaccess's FDA-ready data, you can achieve patient enrollment 50% quicker, saving approximately $25K per patient, which highlights the significance of employing advanced tools in overseeing projects.
Conduct Regular Risk Assessments: Identify potential risks early and develop contingency plans. Regularly reviewing these evaluations during the process can help mitigate problems before they escalate. Research indicates that effective project management in clinical trials considerably decreases risks and errors, highlighting the importance of proactive oversight. Bioaccess's expertise in study setup and compliance evaluations can further assist this process by ensuring that all regulatory requirements are fulfilled.
Engage Stakeholders: Maintain open lines of communication with all stakeholders, including sponsors, regulatory bodies, and site staff. Regular updates and feedback loops can foster collaboration and ensure alignment on objectives. Efficient communication can prevent misunderstandings and enhance the overall administration of medical studies, resulting in improved outcomes. As noted by Halloran Consulting Group, a skilled program manager can save more than their total compensation cost, underscoring the importance of expertise in overseeing projects. Bioaccess's initiative oversight and monitoring services guarantee that all stakeholders remain informed and involved throughout the study.
By applying these strategies, study managers can enhance their efficiency, manage expenses, and adhere to schedules, ultimately fostering the success of their research. Significantly, organizations that adopt project management in clinical trials achieve a 92% success rate in meeting project objectives, whereas only one in five attempts is completed on schedule, demonstrating the critical importance of project management in addressing challenges.
Ensuring adherence to regulatory standards and protocols is paramount for project management in clinical trials. Key practices to follow include:
By investing in robust SOPs and adopting a proactive strategy for compliance, organizations can significantly improve project management in clinical trials, ultimately facilitating the creation of safe and effective treatments.

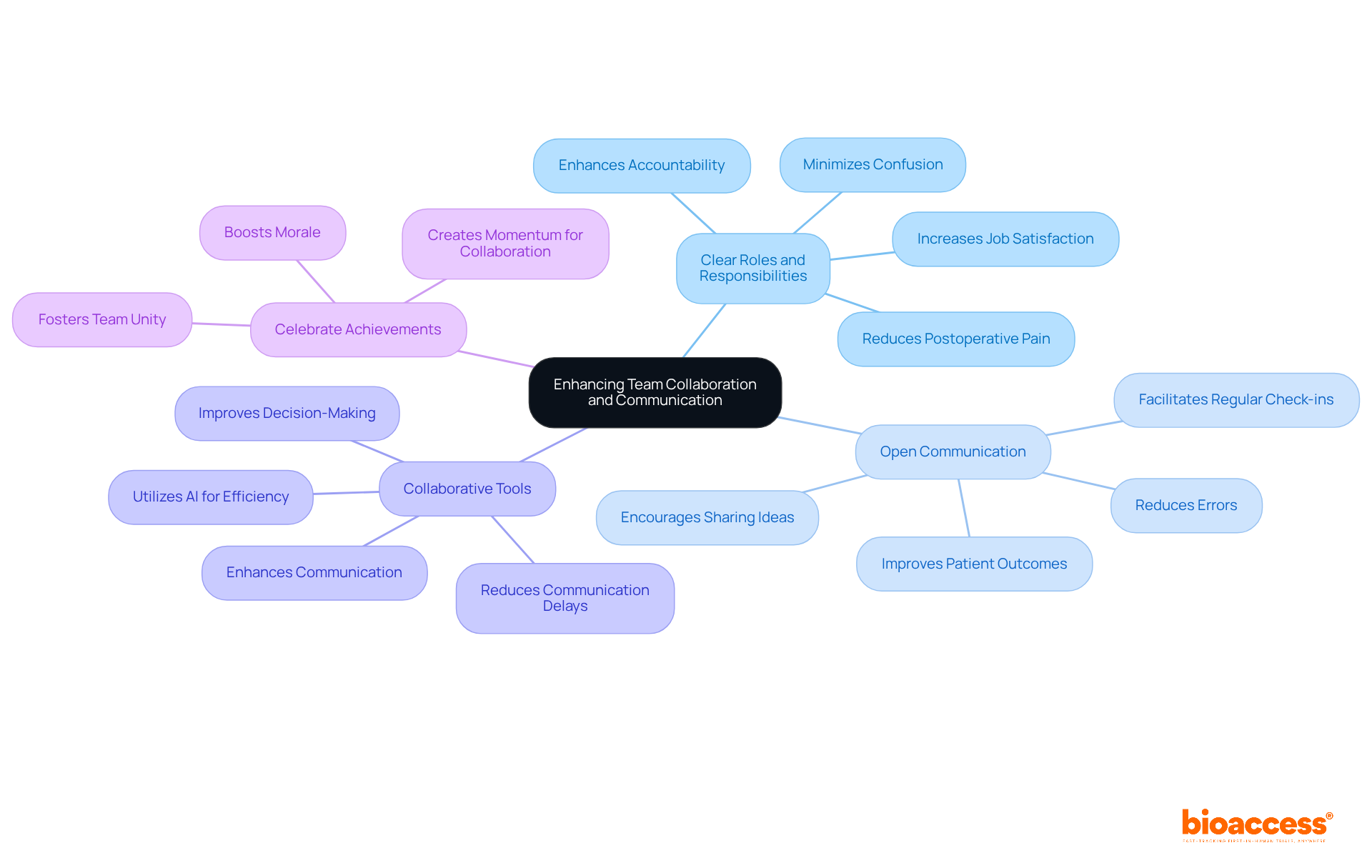
Nurturing a cooperative atmosphere is vital for the success of medical studies. To enhance team collaboration and communication, consider the following strategies:
Establish Clear Roles and Responsibilities: Clearly defining each team member's role and responsibilities minimizes confusion and overlap. This streamlining of processes enhances accountability. Studies suggest that enhanced role clarity within multidisciplinary teams is linked to increased job satisfaction, a crucial factor in high-pressure settings such as research trials. Additionally, patients from teams characterized by higher levels of role clarity, mutual trust, and quality information exchange experience lower levels of postoperative pain and shorter lengths of stay (Gittell et al., 2000).
Encourage Open Communication: Cultivating an environment where team members feel comfortable sharing ideas, concerns, and feedback is essential. Regular team meetings and check-ins facilitate open dialogue, which correlates with improved patient outcomes and reduced errors in clinical settings. Notably, studies indicate that approximately 30% of team interactions in surgical services involve a communication failure (Lingard et al., 2004), underscoring the necessity of fostering open communication among team members.
Utilize Collaborative Tools: Implementing tools that enhance communication and collaboration—such as shared document platforms, messaging apps, and project management software—ensures alignment among all team members. Real-time collaboration tools can significantly reduce communication delays, leading to quicker decision-making and enhanced efficiency. Moreover, AI examines testing data to highlight possible problems or inefficiencies, demonstrating how technology can enhance communication and decision-making.
Celebrate Team Achievements: Recognizing and celebrating milestones fosters a sense of unity and boosts morale among team members. This recognition not only motivates the team but also reinforces the importance of teamwork in the context of project management in clinical trials to achieve objectives. Celebrating small wins can create momentum for ongoing collaboration and success.
Mastering project management in clinical trials is essential for achieving successful outcomes in an environment fraught with unique challenges. Understanding these challenges—ranging from regulatory compliance to patient recruitment and budget management—is crucial for any organization aiming to navigate the complexities of clinical trials effectively. By employing strategic project management techniques, organizations can significantly enhance their chances of success.
Key insights highlight the importance of:
These strategies not only streamline processes but also foster collaboration and communication among team members, which is vital for maintaining project momentum and ensuring adherence to regulatory standards. Organizations that implement robust project management practices can achieve a remarkable success rate in meeting their objectives.
In a landscape where the stakes are high and the margin for error is slim, the call to action is clear: prioritize effective project management in clinical trials. By embracing best practices and leveraging expert support, organizations can overcome barriers, enhance operational efficiency, and ultimately contribute to the development of safe and effective treatments. The future of clinical trials depends on the commitment to mastering these essential project management skills, ensuring that the path from research to real-world application is as smooth and successful as possible.
What are the unique challenges of project management in clinical trials?
The unique challenges include regulatory compliance, patient recruitment and retention, budget constraints, and data handling issues.
Why is regulatory compliance important in clinical trials?
Regulatory compliance is critical because each jurisdiction has its own requirements, and non-compliance can lead to expensive delays or cancellations of studies. Companies with strong compliance abilities experience 62% fewer serious adverse occurrences in research studies.
What services does bioaccess™ offer for clinical trial project management?
bioaccess™ offers services such as feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
How does patient recruitment impact clinical trials?
Inadequate enrollment is the primary reason for study terminations, with 55% of investigations halted due to weak recruitment. Factors affecting recruitment include stringent eligibility criteria, patient awareness, and willingness to participate.
Can you provide an example of successful patient recruitment in clinical trials?
GlobalCare Clinical Studies collaborated with bioaccess™ to enhance its research ambulatory services in Colombia, achieving over a 50% decrease in recruitment time and a retention rate exceeding 95%.
What role does budget management play in clinical trials?
Effective budget management is essential to prevent budget excesses that can jeopardize the entire project, as conducting experiments involves elevated expenses that require meticulous financial oversight.
Why is data handling critical in clinical trials?
Maintaining data integrity and security is crucial, as ineffective data oversight can lead to errors that jeopardize the legitimacy of the study. Automated systems for regulatory tracking can significantly reduce compliance gaps.
How can early identification of challenges benefit clinical trial project management?
By identifying challenges early, managers can develop proactive strategies to address them efficiently, leading to more successful studies.
Who are some professionals that contribute to effective clinical trial project management?
Professionals such as Ana Criado and Katherine Ruiz contribute to ensuring that research studies are administered with high standards of regulatory compliance and operational efficiency.