


The article outlines the essential logistics and regulatory requirements for importing medical devices into Mexico. It highlights the critical nature of:
This collaboration is vital for successfully navigating the complex importation process, ensuring compliance, and achieving timely market entry.
Successfully importing medical devices into Mexico necessitates a nuanced understanding of the regulatory landscape and the logistical intricacies that govern this vital sector. As the healthcare market is projected to thrive, navigating the complexities of importation presents significant opportunities for growth and innovation.
However, with evolving regulations from COFEPRIS and the potential pitfalls of compliance, how can importers ensure a seamless process while maximizing their market potential? This guide explores the essential steps and best practices for mastering purchase importer logistics for medical devices in Mexico, equipping readers with the knowledge necessary to excel in this dynamic environment.
Successfully managing the purchase importer logistics for medical equipment into Mexico necessitates a comprehensive grasp of the importation procedure, particularly the role of the Comisión Federal para la Protección contra Riesgos Sanitarios (COFEPRIS). This regulatory organization supervises the importation of healthcare instruments, ensuring adherence to national health standards. It is essential to familiarize yourself with the various classifications of healthcare devices and their specific import requirements, as these can vary significantly based on the device's intended use.
In 2025, COFEPRIS updated its regulations to streamline the importation process, reflecting the evolving landscape of medical technology. Importation licenses, valid for 180 days, must be applied for electronically through the Digital International Trade Single Window (VUCEM). Additionally, for the purchase importer logistics Mexico medical importation process, a Sanitary Registration Number from COFEPRIS is required. Understanding these updates is crucial for effectively navigating the regulatory environment.
The revised Agreement in the country, effective July 7, 2025, features a new list of low-risk and deregulated items, a significant factor for importers. Successful instances of healthcare equipment importation under COFEPRIS regulations illustrate the importance of effective purchase importer logistics in Mexico for medical equipment, emphasizing the need for a designated Registration Holder (MRH) or licensed distributor. These entities are vital for managing the registration process and ensuring compliance with COFEPRIS requirements. For instance, foreign manufacturers that have partnered with experienced MRHs have successfully navigated the complexities of customs and regulatory approvals related to purchase importer logistics in Mexico for medical products, leading to timely market entry.
To position your products effectively, it is important to analyze market dynamics, including demand and competition. The income of the healthcare tools market in the country is stated to be 8 billion USD, and the total production value of health equipment is 100 billion MXN. Investigating the specific healthcare tools you intend to purchase, along with the importer logistics in Mexico for medical items, and their relevant regulations under Mexican law, particularly the General Health Law, will provide a solid foundation for your import strategy. Furthermore, distinguishing the roles of MRH and distributor is crucial to avoid conflicts of interest and ensure compliance. By leveraging local knowledge and compliance expertise, you can enhance your prospects for successful commercialization in the burgeoning healthcare market of the country.
Before introducing healthcare instruments into Mexico, it is crucial to fully understand the regulatory framework established by COFEPRIS. This entails obtaining a sanitary registration, which confirms that your health instrument complies with the necessary safety and efficacy standards. Additionally, an import permit is mandatory for all healthcare devices entering the country.
Compliance with labeling requirements is vital; instructions for use must be provided in Spanish to adhere to COFEPRIS standards. Moreover, familiarize yourself with the classification of your equipment, as different categories—ranging from low-risk to high-risk—carry varying compliance obligations. For instance:
Staying informed about the latest recommendations from COFEPRIS is essential, especially as the agency has streamlined its processes and introduced expedited systems that can reduce review times by up to 5 months for products already approved by major oversight organizations like the US FDA. Collaborating with local experts can further ease compliance and significantly enhance the likelihood of successful market entry.
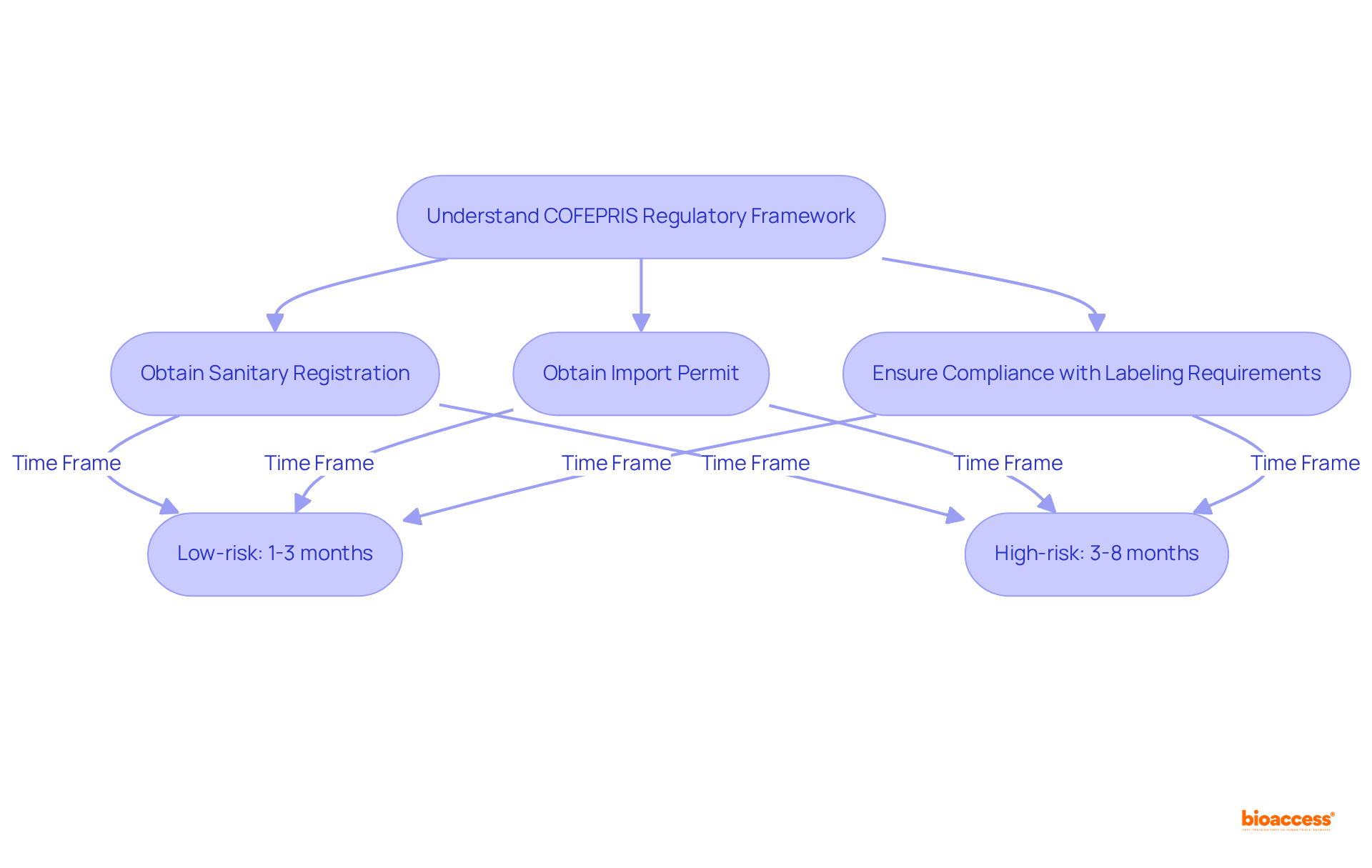
To successfully import medical devices into Mexico, it is imperative to adhere to the following step-by-step logistics procedures:
Obtain Sanitary Registration: Ensure your health instrument is registered with COFEPRIS, the authority responsible for health safety in the country. This registration is essential before applying for an import permit. Considering that imports account for almost 90% of the healthcare instruments sold in Mexico, following the purchase importer logistics for medical supplies is crucial.
Apply for an Import Permit: Submit your application electronically via the Digital International Trade Single Window (VUCEM). This import permit is valid for 180 days, allowing for efficient planning. The regulatory environment can be challenging for purchase importer logistics in Mexico, especially in the medical sector, as COFEPRIS's approval process is often time-consuming, making timely application key.
Prepare Required Documentation: Compile all necessary documents, including the commercial invoice, packing list, and certificates of origin. It is crucial that these documents comply with Mexican regulations to avoid delays. The healthcare equipment sector in Mexico is substantial, with firms providing more than 71,000 positions in areas such as Baja California, highlighting the necessity of adhering to purchase importer logistics in the medical field.
Customs Clearance: Engage a customs broker to facilitate the clearance process. Their expertise will help navigate applicable customs duties and taxes, ensuring a smooth transition through customs. This step is vital as the healthcare equipment manufacturing industry in the country is one of the leading producers globally, and effective purchase importer logistics Mexico medical can enhance your customs clearance and improve your market entry.
Delivery and Distribution: After clearance, arrange the transport of your healthcare products to the specified location in the country. Select a logistics provider skilled in managing healthcare equipment to guarantee adherence to safety standards and regulations. With the healthcare market of the country valued in the multi-billions, effective purchase importer logistics Mexico medical is crucial for capitalizing on this potential.
By adhering to these procedures, companies can effectively handle the intricacies of importing healthcare products into Mexico, utilizing the nation's favorable business environment and regulatory framework.
Selecting the appropriate partners for your purchase importer logistics Mexico medical is essential for guaranteeing a smooth process in the healthcare equipment sector. Here are some essential tips:
Choose a Customs Broker: Collaborate with a customs broker who possesses in-depth knowledge of importing healthcare products. Their expertise is invaluable in navigating the complex regulatory landscape and ensuring compliance with all import requirements. This is particularly vital given the projected growth of the Mexico Customs Brokerage Market, expected to reach USD 345.65 million in 2025. Such growth underscores the increasing demand for skilled customs brokers capable of facilitating efficient import processes.
Logistics Providers: Partner with logistics firms that specialize in healthcare products. These providers in purchase importer logistics Mexico medical must have a proven track record of managing sensitive products and be well-versed in the specific regulations governing medical device transportation. The purchase importer logistics for medical devices in Mexico is projected to grow at a CAGR of 5.02% from 2024 to 2032, indicating a heightened demand for reliable logistics services. Selecting the right purchase importer logistics Mexico medical partner is more critical than ever to ensure compliance and operational efficiency.
Consult Regulatory Experts: Engage with regulatory consultants who can provide insights into the latest changes in import regulations. Their guidance will be crucial in preparing the necessary paperwork, especially as the healthcare equipment market in Mexico is anticipated to expand at an annual rate of 6.31% from 2025 to 2030.
Network with Industry Colleagues: Join industry associations or forums to network with other professionals in the healthcare equipment sector. This networking can yield valuable resources and recommendations for trustworthy partners, fostering successful collaborations that enhance your logistics operations. Strong partnerships with logistics providers are essential for ensuring the timely and compliant transportation of medical devices, ultimately contributing to the efficiency of your supply chain.
Successfully navigating the complexities of purchasing importer logistics for medical devices in Mexico is essential for any business seeking to enter this lucrative market. Understanding the regulatory landscape, particularly the pivotal role of COFEPRIS, forms the cornerstone of an effective import strategy. By familiarizing oneself with the importation process—including obtaining necessary registrations and permits—companies can ensure compliance and streamline their market entry.
Key insights from the article underscore the importance of adhering to regulatory requirements, such as:
Collaborating with experienced partners, like customs brokers and logistics providers, is crucial for overcoming the challenges inherent in medical device importation. Moreover, leveraging local expertise can enhance compliance and facilitate a smoother import process, ultimately leading to successful commercialization in Mexico's burgeoning healthcare sector.
In conclusion, the medical device importation landscape in Mexico presents both opportunities and challenges. Businesses must prioritize understanding the regulatory framework and establishing reliable partnerships to thrive in this competitive environment. By implementing best practices and staying informed about evolving regulations, companies can position themselves for success and capitalize on the significant growth potential within Mexico's healthcare market. Taking proactive steps today will pave the way for a successful tomorrow in medical device import logistics.
What is the role of COFEPRIS in the importation of medical devices in Mexico?
COFEPRIS, the Comisión Federal para la Protección contra Riesgos Sanitarios, supervises the importation of healthcare instruments in Mexico, ensuring adherence to national health standards.
What are the key updates to the importation regulations by COFEPRIS in 2025?
In 2025, COFEPRIS updated its regulations to streamline the importation process, including the requirement for electronic applications for importation licenses through the Digital International Trade Single Window (VUCEM) and the necessity of a Sanitary Registration Number from COFEPRIS.
How long are the importation licenses valid for medical devices in Mexico?
Importation licenses for medical devices in Mexico are valid for 180 days.
What is the significance of the revised Agreement effective July 7, 2025?
The revised Agreement includes a new list of low-risk and deregulated items, which is significant for importers as it affects the classification and regulatory requirements for various medical devices.
Why is it important to have a designated Registration Holder (MRH) or licensed distributor in Mexico?
An MRH or licensed distributor is vital for managing the registration process and ensuring compliance with COFEPRIS requirements, which is essential for successful importation of medical equipment.
What are the financial figures related to the healthcare tools market in Mexico?
The income of the healthcare tools market in Mexico is approximately 8 billion USD, and the total production value of health equipment is around 100 billion MXN.
What should importers analyze to position their products effectively in the Mexican market?
Importers should analyze market dynamics, including demand and competition, as well as the specific healthcare tools they intend to purchase and the relevant regulations under Mexican law, particularly the General Health Law.
How can leveraging local knowledge and compliance expertise benefit importers in Mexico?
Leveraging local knowledge and compliance expertise can enhance the prospects for successful commercialization in the growing healthcare market in Mexico by ensuring adherence to regulations and effective management of the importation process.