


This article highlights the essential practices for mastering Quality Assurance (QA) in the medical devices sector, underlining its critical role in ensuring patient safety and regulatory compliance. It elaborates on key components such as document control, risk management, and the crucial distinction between QA and Quality Control (QC). By demonstrating how robust QA practices can significantly enhance product reliability, it effectively illustrates the importance of mitigating risks in healthcare offerings.
Quality assurance in the medical device industry is not merely a regulatory requirement; it is a critical component that safeguards patient safety and enhances the reliability of healthcare products. As organizations strive to navigate the complexities of compliance and innovation, understanding the key practices that underpin effective QA becomes essential.
The challenges manufacturers face in balancing proactive quality assurance with reactive quality control are significant. How can they leverage best practices to mitigate risks and ensure market success? This exploration is vital for fostering a robust Medtech landscape.
Quality Assurance (QA) medical devices encompass the systematic processes and procedures that ensure these instruments adhere to specified requirements and standards throughout their lifecycle. This discipline is vital in the QA medical devices sector, as it not only prevents flaws but also ensures compliance with regulatory standards to ultimately safeguard patient well-being. In Colombia, the National Food and Drug Surveillance Institute (INVIMA) plays a pivotal role in this arena, overseeing the marketing and manufacturing of health goods, including healthcare instruments. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures that QA medical devices meet rigorous safety, efficacy, and quality standards.
Effective QA medical devices practices significantly enhance the reliability of healthcare offerings and cultivate trust among providers and patients. For instance, 96% of organizations reported experiencing a recall of goods in the past five years, underscoring the necessity of robust QA systems. By implementing comprehensive QA medical devices processes, organizations can streamline development timelines and enhance market competitiveness, enabling innovative solutions to reach patients more swiftly and safely. Recent updates, such as the European Commission's 2025 Guidance on healthcare product regulations, further illuminate the evolving landscape of QA medical devices, necessitating that manufacturers adapt to ensure compliance and capitalize on growth opportunities. Moreover, case studies reveal that 83% of organizations believe a quality management solution aided their recovery from recalls, highlighting the strategic importance of QA in mitigating risks and enhancing overall safety.
Additionally, bioaccess® provides extensive clinical trial management services, including:
These services are crucial for Medtech, Biopharma, and Radiopharma startups seeking to navigate the regulatory landscape effectively and expedite their clinical trials. Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, underscores the necessity of aligning QA medical devices practices with regulatory requirements to ensure successful market entry and patient safety.

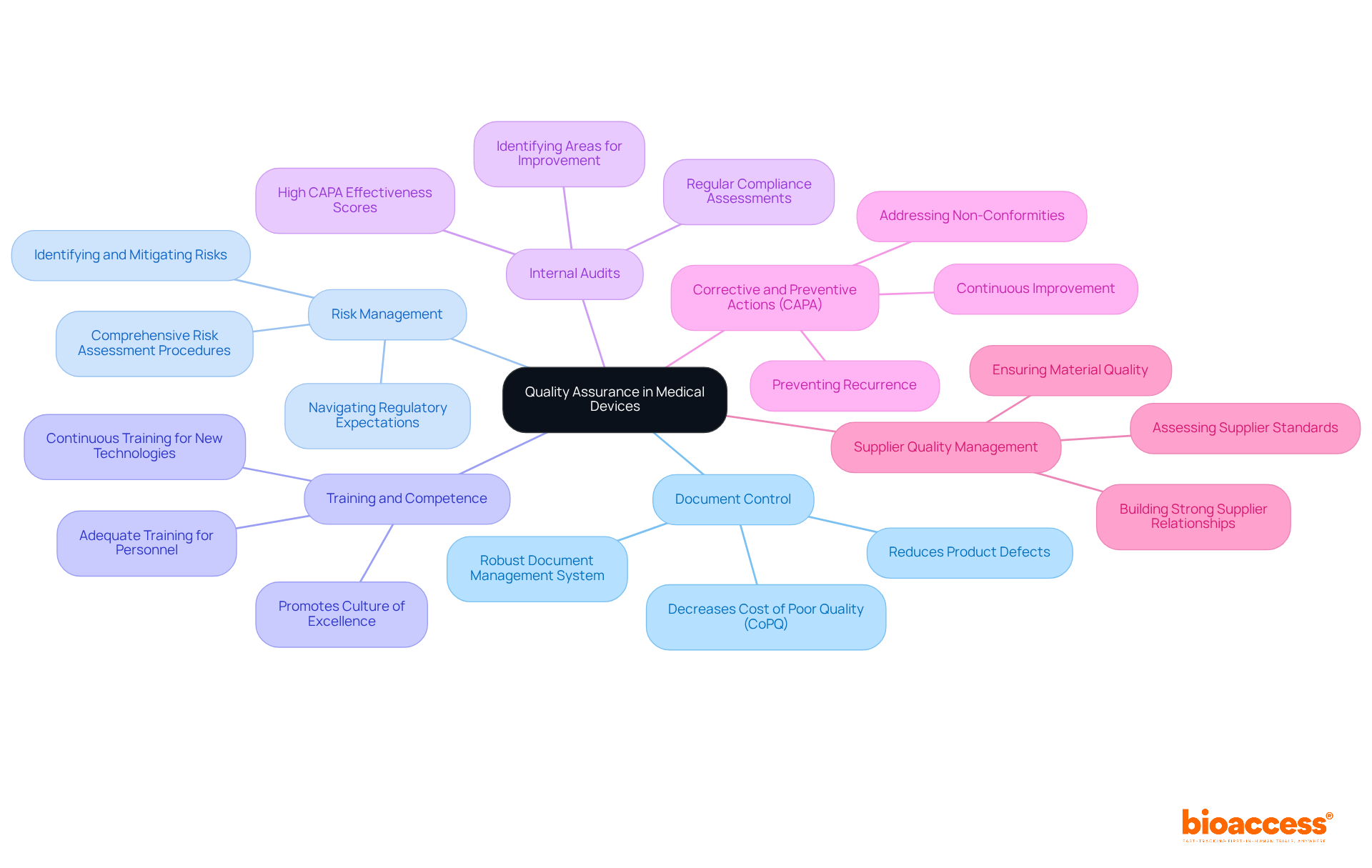
Key components of Quality Assurance (QA) in medical devices include:
Document Control: A robust document management system is essential to ensure that all quality-related documents are current, accessible, and properly archived. Effective document control can reduce product defects by taking proactive steps early in the design-to-production process, leading to a significant decrease in total Cost of Poor Quality (CoPQ). Preventing defects can often halve total Cost of Poor Quality (CoPQ).
Risk Management: Implementing comprehensive risk assessment procedures is crucial for identifying, evaluating, and mitigating potential risks associated with medical devices throughout their lifecycle. A documented risk assessment can provide essential insights and help organizations navigate regulatory expectations effectively.
Training and Competence: Ensuring that all personnel involved in the QA process are adequately trained promotes a culture of excellence within the organization. As manufacturing environments evolve rapidly, continuous training is vital for maintaining high standards and adapting to new technologies.
Internal Audits: Regular internal audits are necessary to assess compliance with QA procedures and identify areas for improvement. Organizations with high Corrective and Preventive Action (CAPA) effectiveness scores typically experience recall rates of less than 0.2% of shipped units, highlighting the importance of thorough audits.
Corrective and Preventive Actions (CAPA): Establishing a CAPA system to address non-conformities and prevent recurrence is essential for continuous improvement in QA processes. This proactive strategy guarantees that problems are addressed efficiently, improving overall standards.
Supplier Quality Management: Assessing and overseeing suppliers is essential to guarantee that materials and components satisfy standards before incorporation into the final item. A strong supplier relationship focused on constant improvement can significantly enhance the quality of the end product.
By implementing these components, organizations can establish a thorough QA framework for QA medical devices that supports the development of safe and effective healthcare products, ultimately leading to enhanced patient outcomes and regulatory compliance.
Quality Assurance (QA) medical devices and Quality Control (QC) are often conflated, yet they serve distinct roles in the development of healthcare products.
Quality Assurance represents a proactive strategy focused on defect prevention through systematic processes and standards. It encompasses the entire lifecycle of QA medical devices—from design and production to post-market surveillance—ensuring compliance with regulatory requirements. This proactive approach is vital, as it not only mitigates risks but also cultivates a culture of continuous improvement. As industry leaders assert, 'Quality is never an accident; it is always the result of high intention, sincere effort, intelligent direction, and skillful execution.' Statistics reveal that adopting a Design for Manufacturability approach can significantly reduce defects by addressing issues early in the design-to-production continuum, underscoring the critical role of QA.
Conversely, Quality Control is a reactive process that entails inspecting and testing products to identify defects post-production. QC activities are conducted at various manufacturing stages to confirm that the final product meets established standards of excellence. This reactive nature is essential for promptly addressing any issues, thereby safeguarding patient safety and product efficacy.
Understanding the distinction between QA medical devices and QC is crucial for organizations aiming to implement robust management systems. While QA focuses on preventing problems before they arise, QC is dedicated to identifying and rectifying flaws, ensuring that health products are safe and effective for patient use.
Real-world examples highlight this dynamic: organizations that emphasize QA often experience fewer recalls and defects, whereas those that depend predominantly on QC may incur higher costs and delays due to late-stage problem identification. For instance, a case study on integrated management of standards illustrates that organizations adopting a comprehensive approach to excellence across all functions are more likely to achieve superior results and enhance overall outcomes. In the competitive realm of medical device development, mastering both QA medical devices and QC is imperative for attaining quality excellence and ensuring regulatory compliance.
However, organizations must also recognize common pitfalls in implementing these practices. Companies that focus excessively on QC without sufficient QA may face significant challenges, including increased operational costs and potential threats to patient safety. Therefore, a balanced strategy that integrates both QA and QC is essential for success.

Mastering quality assurance (QA) in medical devices is crucial for ensuring that healthcare products are safe, effective, and compliant with regulatory standards. This discipline not only mitigates risks but also fosters trust between manufacturers and patients, ultimately enhancing patient outcomes. The systematic processes involved in QA are designed to prevent defects throughout the product lifecycle, underscoring the necessity of a proactive approach in an industry where quality cannot be compromised.
The article emphasizes several key components essential for effective QA in medical devices, including:
Each of these elements plays a pivotal role in establishing a robust QA framework that supports the development of safe healthcare products. Moreover, understanding the distinction between quality assurance and quality control is vital for organizations aiming to implement comprehensive management systems that prioritize both prevention and correction of defects.
In conclusion, the significance of quality assurance in the medical device industry cannot be overstated. As regulatory landscapes evolve and the demand for reliable healthcare products increases, organizations must adopt best practices in QA to ensure compliance while driving innovation and improving patient safety. Embracing a culture of quality and continuous improvement will be essential for organizations striving for excellence in this competitive field. It is imperative for stakeholders to prioritize QA strategies, as they lay the foundation for sustainable success and ultimately safeguard the well-being of patients worldwide.
What is Medical Device Quality Assurance (QA)?
Medical Device Quality Assurance (QA) refers to the systematic processes and procedures that ensure medical devices meet specified requirements and standards throughout their lifecycle, preventing flaws and ensuring compliance with regulatory standards.
Why is QA important in the medical devices sector?
QA is crucial in the medical devices sector because it safeguards patient well-being by ensuring that devices are safe, effective, and of high quality, ultimately building trust among healthcare providers and patients.
What role does INVIMA play in medical device QA in Colombia?
The National Food and Drug Surveillance Institute (INVIMA) oversees the marketing and manufacturing of health goods, including healthcare instruments, ensuring that QA medical devices meet rigorous safety, efficacy, and quality standards.
What percentage of organizations reported experiencing product recalls in the past five years?
96% of organizations reported experiencing a recall of goods in the past five years, highlighting the necessity of robust QA systems.
How can effective QA practices impact healthcare organizations?
Effective QA practices enhance the reliability of healthcare offerings, streamline development timelines, improve market competitiveness, and enable innovative solutions to reach patients more swiftly and safely.
What recent updates are influencing the QA landscape for medical devices?
The European Commission's 2025 Guidance on healthcare product regulations is a recent update that necessitates manufacturers to adapt their practices to ensure compliance and capitalize on growth opportunities.
What percentage of organizations believe that a quality management solution helped them recover from recalls?
83% of organizations believe that a quality management solution aided their recovery from recalls, indicating the strategic importance of QA in mitigating risks and enhancing overall safety.
What clinical trial management services does bioaccess® provide?
Bioaccess® provides services including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, which are crucial for Medtech, Biopharma, and Radiopharma startups.
Why is it important to align QA practices with regulatory requirements?
Aligning QA practices with regulatory requirements is essential to ensure successful market entry and patient safety, as emphasized by Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia.