


The article addresses the essential concepts and challenges associated with randomization in clinical trials, highlighting its critical role in eliminating selection bias and ensuring comparability among treatment groups. It outlines various methodologies for randomization, discusses the implementation challenges that researchers encounter, and emphasizes the ethical and regulatory considerations necessary for upholding the integrity of clinical trials. This underscores the vital importance of proper randomization in producing reliable and valid study outcomes.
Randomization serves as a cornerstone of clinical trials, revolutionizing how researchers allocate participants to treatment groups. This process ensures that outcomes remain unbiased by pre-existing differences. This article delves into the intricacies of randomization, examining its essential methodologies, the challenges encountered during implementation, and the ethical considerations that govern this critical aspect of clinical research. As the landscape of medical studies evolves, researchers must navigate the complexities of randomization to uphold the integrity of their findings while adhering to rigorous ethical standards.
Randomization in clinical trial is a crucial process that entails allocating individuals to various treatment categories solely by chance. This technique is vital for ensuring randomization in clinical trial, which helps eliminate selection bias and ensures that the cohorts are comparable at the beginning of the experiment. Researchers can confidently link any differences in outcomes directly to the interventions being tested through randomization in clinical trial, rather than to pre-existing disparities among the participants. The randomization in clinical trial enhances the validity of study outcomes, making them more reliable for guiding clinical practice and policy decisions.
For instance, in a study assessing a new medication compared to a placebo, the randomization in clinical trial process ensures that both groups are similar in demographics and health status, effectively isolating the drug's effect on patient outcomes. Such rigorous methodologies, including randomization in clinical trial, uphold the integrity of clinical research, as evidenced by the significant advancements in treatment outcomes seen in studies over the past decades.
Thorough clinical study management services, like those offered by bioaccess, are essential in facilitating effective allocation. These services, which include feasibility studies, site selection, compliance reviews, and setup, ensure that studies are well-structured and compliant with regulations. By enhancing the reliability of allocation processes, bioaccess contributes to improved healthcare outcomes and fosters economic advantages through job creation and global cooperation.
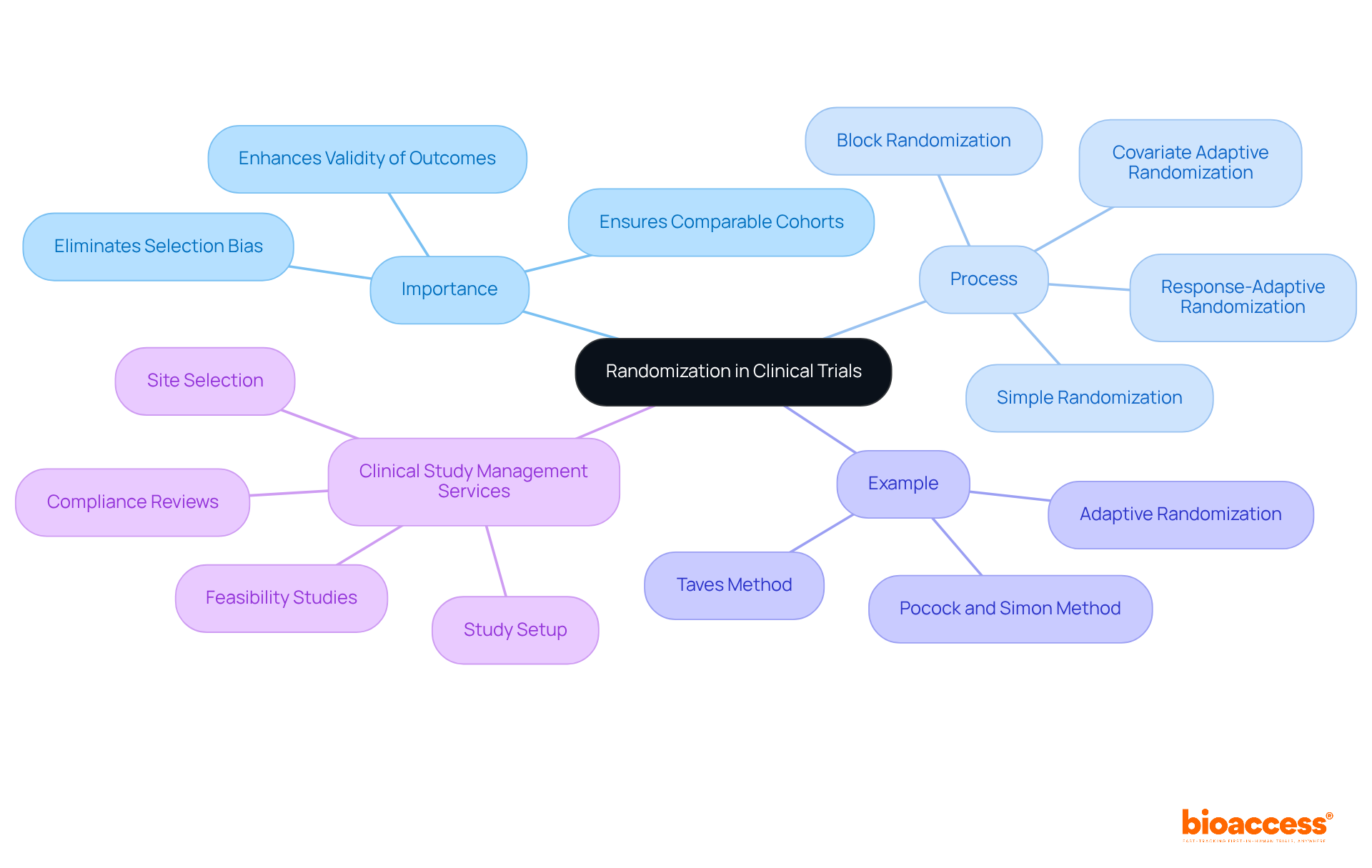
Several methodologies for randomization in clinical trials exist, each with distinct advantages and disadvantages:
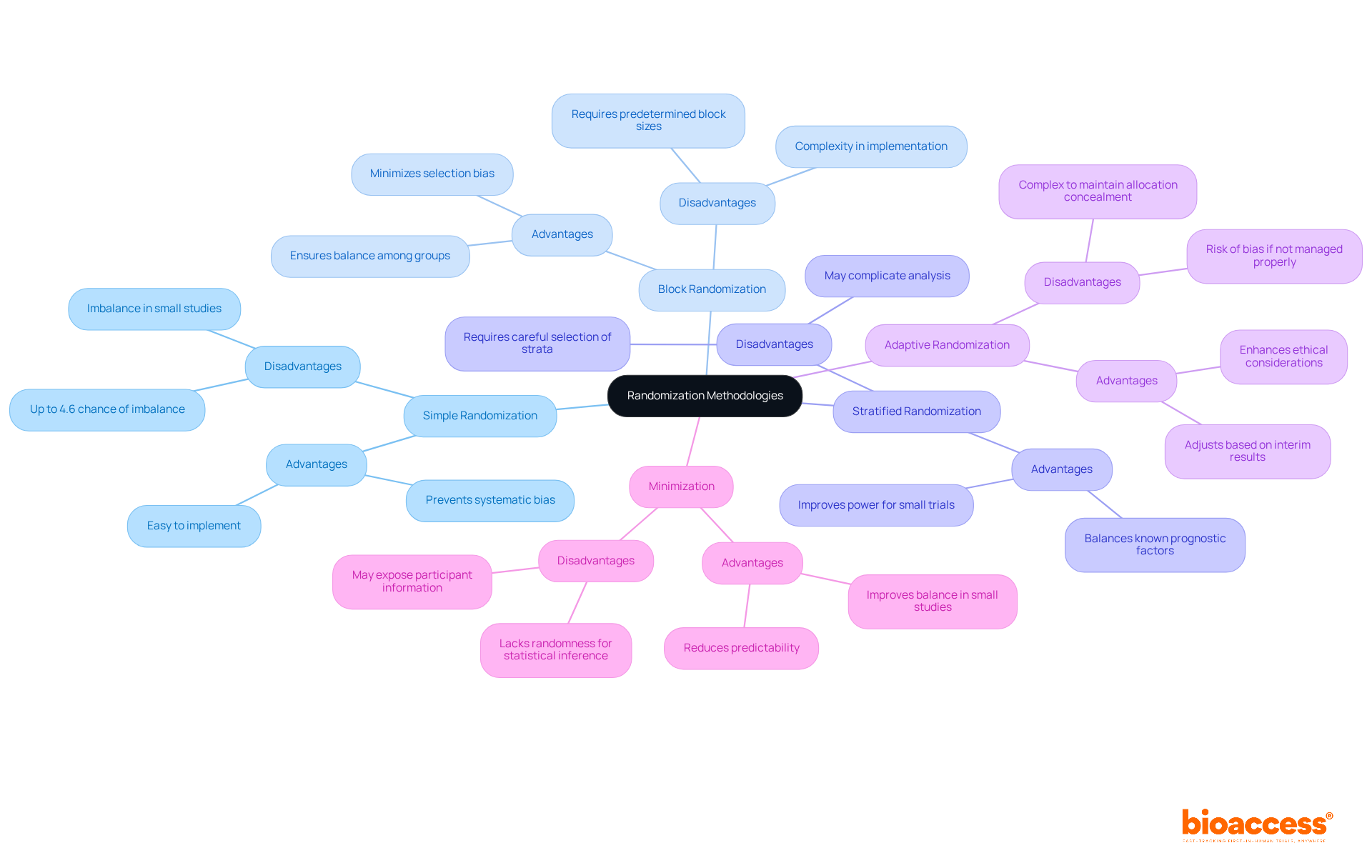
Simple Randomization: This straightforward method provides each individual an equal opportunity of being allocated to any category. While simple to execute, it can result in disparities in participant sizes, especially in smaller studies, where the likelihood of imbalance can reach up to 4.6% for 400 subjects. Minsoo Kang highlights that randomization in clinical trial is regarded by most researchers as the best method for participant allocation in clinical studies since it enhances the outcomes and data analysis.
Block Randomization: Participants are divided into blocks of a predetermined size, ensuring that each treatment category is filled before proceeding to the next block. This approach aids in preserving equilibrium among participants during the experiment, minimizing the chance of selection bias. The effectiveness of this method is highlighted in various studies, which show its utility in preventing imbalances.
Stratified Randomization: In this approach, participants are categorized into strata based on specific characteristics (e.g., age, gender) prior to randomization. This guarantees that these traits are uniformly spread among treatment groups, enhancing comparability and possibly boosting power for small studies. Research indicates that stratification may prevent type I error and improve power for small trials with less than 400 patients.
Adaptive Randomization: This dynamic method allows for adjustments in the randomization process based on interim results. For instance, if one treatment shows greater effectiveness, more participants may be assigned to that group. This approach can enhance both the ethical and scientific value of the study, although it may introduce complexities in maintaining allocation concealment. Ethical factors related to random assignment are essential, as they can influence the integrity of the study.
Minimization: This technique assigns participants to groups in a manner that minimizes the imbalance of certain characteristics across groups. Especially advantageous in small studies, minimization can attain improved balance compared to basic random selection, although it may lack the randomness necessary for statistical inference. A case study on minimizing predictability while maintaining balance through less restrictive allocation procedures illustrates the practical application of this method.
Choosing the suitable allocation technique is essential for the success of a clinical study, as it directly impacts the effectiveness of randomization in clinical trial outcomes. Specialists stress that although basic random methods are efficient for larger studies, block and stratified techniques are better for smaller investigations to avoid imbalances that could distort results. Moreover, over 80% of participants in a recent survey recognized difficulties in implementing new allocation techniques and pinpointed opportunities for enhancement, such as improved education and the creation of standards.
Implementing randomization in clinical trial processes presents several challenges, particularly for medical device startups navigating a complex landscape.
Accidental unblinding is a significant concern, occurring when subjects or researchers unintentionally become aware of treatment assignments, potentially skewing results. To mitigate this risk, robust blinding procedures must be established.
Recruitment issues also pose challenges, as difficulties in enlisting individuals can lead to disparities in group sizes, especially for startups facing apathy from clinical research locations. To address this, researchers should develop a comprehensive recruitment strategy that includes outreach and engagement with potential participants, leveraging accelerated patient recruitment services to enhance efficiency.
Data management is crucial for maintaining the integrity of the process. Ensuring accurate and secure data handling can be achieved by utilizing electronic data capture systems, which streamline the process and reduce errors—an essential consideration given the limited financial resources often faced by startups.
Compliance with protocols is paramount. Adhering to the assignment protocol requires regular training sessions for staff and clear communication of the protocol to ensure compliance, particularly in a convoluted regulatory environment.
Monitoring and auditing are necessary for ongoing observation of the allocation process, assisting in the early detection and correction of problems. Conducting regular audits guarantees that the process is executed as intended, addressing the scrutiny that accompanies regulatory compliance.
Best practices for effective randomization in clinical trials include:

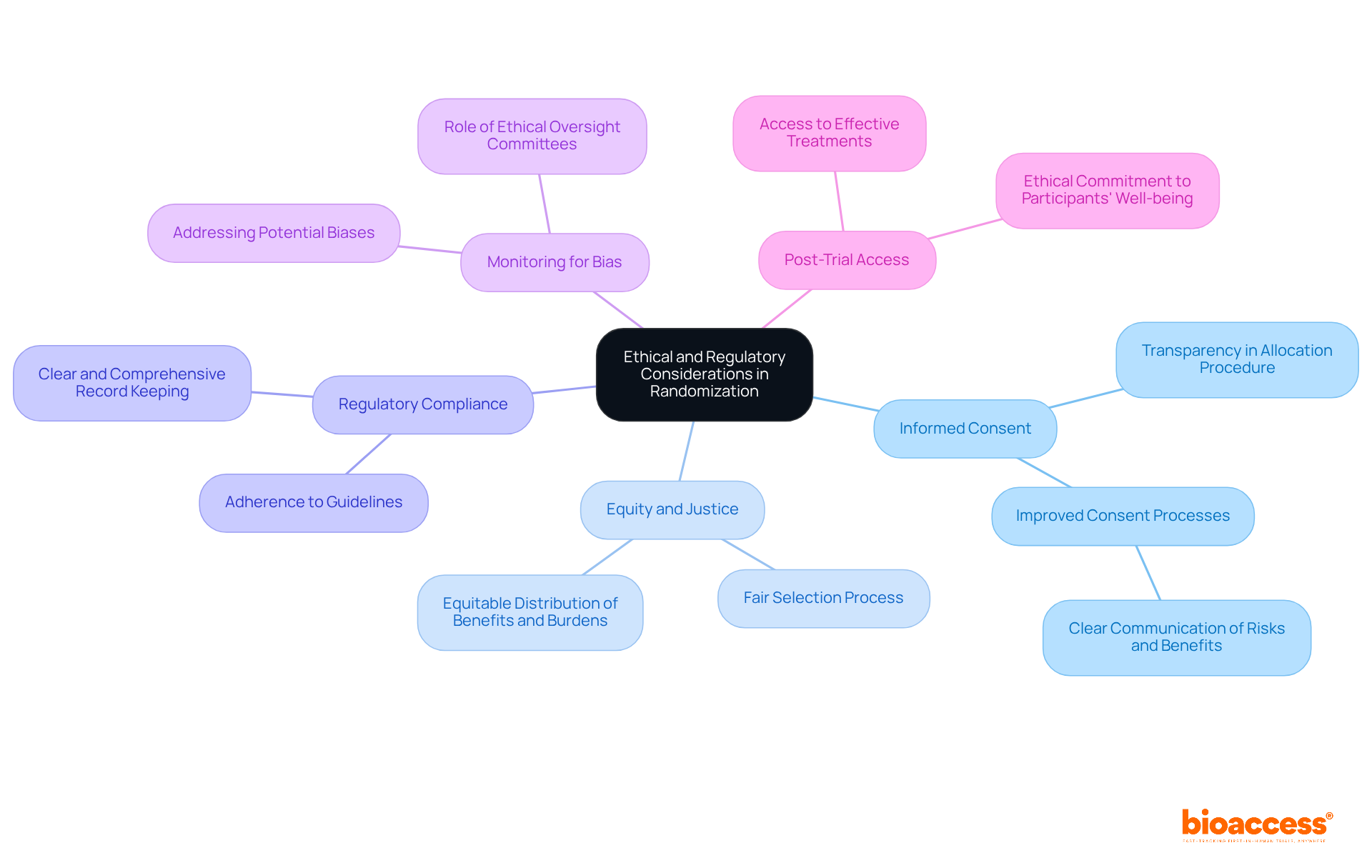
The process of randomization in clinical trial involves critical ethical and regulatory considerations that must be meticulously addressed.
Informed Consent is paramount; individuals must receive comprehensive details about the study, including the allocation procedure. This transparency empowers them to make informed decisions regarding their involvement. Research indicates that only 1.6% of phase I oncology studies utilized risk-targeting designs, underscoring the need for improved consent processes that clearly communicate potential risks and benefits.
Equity and Justice dictate that the selection process must be executed fairly, ensuring that no group faces undue disadvantage. This principle involves the equitable distribution of benefits and burdens across diverse populations, aligning with ethical standards that prioritize justice in clinical research.
Regulatory Compliance is essential, as adherence to guidelines established by regulatory authorities, such as the FDA and EMA, is mandatory. These regulations require that the allocation process be clear and comprehensively recorded, safeguarding the integrity of the study and the rights of participants. At bioaccess, our extensive clinical study management services encompass feasibility assessments, site selection, and compliance evaluations to ensure adherence to these regulations.
Monitoring for bias is crucial; ethical oversight committees are tasked with overseeing studies to ensure the proper implementation of randomization in clinical trial. They are responsible for recognizing and addressing any potential biases that may arise during the study, thereby preserving its validity. Our project management services at bioaccess facilitate ongoing monitoring and reporting, ensuring that any issues are swiftly identified and resolved.
Post-Trial Access extends ethical considerations beyond the study's duration. Researchers must contemplate how individuals, particularly those assigned to control groups, will obtain effective treatments after the study concludes. This aspect is vital for maintaining the ethical commitment to the well-being of those involved. Bioaccess is dedicated to assisting researchers in managing these complexities, ensuring that the well-being of individuals remains a priority throughout the study process.
By rigorously addressing these ethical and regulatory considerations, researchers can uphold the integrity of their trials, protect participant rights, and significantly contribute to the advancement of medical knowledge.
Mastering randomization in clinical trials is essential for ensuring the integrity and reliability of research outcomes. By allocating participants to different treatment groups purely by chance, researchers effectively eliminate selection bias, allowing for the attribution of any observed differences in outcomes directly to the interventions being tested. This foundational concept not only enhances the validity of clinical findings but also strengthens the evidence base that informs clinical practice and policy decisions.
The article explored several key methodologies of randomization, including:
Each method presents unique advantages and challenges, emphasizing the importance of selecting the appropriate allocation strategy based on the specific context of the study. Additionally, practical challenges in implementing randomization, particularly for medical device startups, were addressed, highlighting the necessity for robust planning, stakeholder engagement, and compliance with ethical and regulatory standards.
The significance of randomization extends beyond individual studies; it is a critical component that upholds the ethical framework of clinical research. As the landscape of clinical trials continues to evolve, researchers are urged to prioritize transparency, participant welfare, and adherence to regulatory guidelines. By doing so, they not only enhance the quality of their research but also contribute to the broader goal of advancing medical knowledge and improving patient outcomes. Embracing these principles will ensure that clinical trials remain a cornerstone of evidence-based medicine.
What is randomization in clinical trials?
Randomization in clinical trials is the process of allocating individuals to different treatment groups purely by chance, which helps eliminate selection bias and ensures that the groups are comparable at the start of the experiment.
Why is randomization important in clinical trials?
Randomization is important because it allows researchers to attribute differences in outcomes directly to the interventions being tested, rather than to pre-existing differences among participants, thereby enhancing the validity and reliability of study results.
How does randomization affect the validity of study outcomes?
By ensuring that treatment groups are similar in demographics and health status, randomization helps isolate the effects of the intervention being studied, making the outcomes more reliable for informing clinical practice and policy decisions.
Can you provide an example of randomization in clinical trials?
In a study comparing a new medication to a placebo, randomization ensures that both groups are similar in terms of demographics and health status, which helps accurately assess the drug's effect on patient outcomes.
What role do clinical study management services play in randomization?
Clinical study management services, such as those provided by bioaccess, facilitate effective allocation in trials through feasibility studies, site selection, compliance reviews, and setup, ensuring that studies are well-structured and compliant with regulations.
How does bioaccess contribute to randomization in clinical trials?
Bioaccess enhances the reliability of allocation processes in clinical trials, which contributes to improved healthcare outcomes and fosters economic advantages through job creation and global cooperation.