


The article underscores the mastery of regulatory affairs in pharmaceuticals as essential for achieving compliance success. It highlights the pivotal role of professionals in ensuring adherence to the regulations that govern drug development and marketing. By outlining key strategies—such as thorough documentation, ongoing training, risk management, and leveraging technology—the article illustrates how these elements enhance compliance and operational efficiency in an increasingly complex regulatory landscape.
The pharmaceutical industry is navigating an increasingly complex regulatory landscape, where compliance stands as not merely a requirement but a cornerstone of operational success. As the global market for regulatory compliance is poised for significant growth, grasping the intricate role of regulatory affairs becomes essential for professionals dedicated to ensuring that pharmaceutical products are safe, effective, and market-ready. However, with regulations evolving and the rapid pace of technological advancement, organizations must consider:
The essential processes and activities that ensure compliance with regulations governing the development, testing, and marketing of pharmaceutical products are encompassed by regulatory affairs in pharmaceuticals. Regulatory affairs in pharmaceuticals serve as a vital link between the pharmaceutical industry and regulatory bodies, guaranteeing that products are safe, effective, and of high quality.
Professionals in regulatory affairs in pharmaceuticals are tasked with:
Their role is critical in navigating the intricate landscape of drug development, particularly in regulatory affairs in pharmaceuticals, as the global pharmaceutical compliance market is projected to reach USD 20.56 billion by 2033, expanding at a compound annual growth rate (CAGR) of 8.10% from 2025 to 2033. This growth underscores the increasing complexity of compliance within regulatory affairs in pharmaceuticals and the necessity for robust oversight strategies.
Effective compliance strategies within pharmaceutical firms involve:
Expert opinions highlight that efficient regulatory affairs in pharmaceuticals extend beyond merely fulfilling requirements; they also foster innovation and ensure timely market access for new therapies. As the landscape continues to evolve, the importance of regulatory affairs in pharmaceuticals remains paramount, driving the success of drug development initiatives.

To achieve compliance and efficiency in regulatory affairs in pharmaceuticals, organizations must adopt best practices that are not only effective but also essential in today's complex landscape.
Thorough Documentation: Maintaining comprehensive records of all compliance submissions, communications, and activities is crucial. This practice ensures transparency and facilitates audits. Organizations with structured documentation processes report a remarkable 63% reduction in compliance-related incidents, highlighting the importance of thorough record-keeping.
Regular Training: Ongoing staff training on compliance requirements and changes is vital. Organizations with organized analytics education programs have achieved 47% greater user adoption rates and experienced regulatory advantages 68% quicker than those lacking formal training efforts. This underscores the necessity of continuous education in fostering compliance.
Fostering collaboration between teams in regulatory affairs in pharmaceuticals, R&D, quality assurance, and marketing ensures alignment and streamlines processes. This integrated approach significantly enhances operational efficiency and outcomes in regulatory affairs in pharmaceuticals, demonstrating the power of teamwork in achieving compliance.
Risk Management: Establishing a robust risk management framework enables organizations to proactively identify, assess, and mitigate potential regulatory risks. Notably, 83% of risk and regulatory professionals believe that ensuring adherence to relevant laws and regulations is essential for informed decision-making, reinforcing the need for effective risk management strategies.
Continuous Improvement: Consistently examining and revising adherence methods to incorporate feedback and adapt to evolving regulations is essential. Organizations leveraging technology and best practices, such as real-time analytics, can enhance efficiency and improve risk management, ultimately safeguarding their reputation and operations.
Comprehensive Clinical Trial Management: Engage in thorough feasibility studies and site selection to ensure the right principal investigators are chosen. Review and provide feedback on study documents to comply with country requirements, ensuring effective trial setup and approval processes, including obtaining necessary import permits and nationalization of investigational devices. Effective project management and monitoring, along with detailed reporting on study status and adverse events, are crucial for maintaining compliance and efficiency.

Navigating the evolving compliance landscape is crucial for organizations engaged in regulatory affairs in pharmaceuticals, as it helps them remain informed about changes in regulations, guidelines, and industry standards. Organizations must adopt key strategies in regulatory affairs in pharmaceuticals to ensure compliance and mitigate risks associated with violations.
Monitoring Compliance Updates is essential. Regularly reviewing updates from oversight agencies such as the FDA and EMA is crucial for organizations to stay informed about new requirements and guidance in regulatory affairs in pharmaceuticals. This proactive approach not only guarantees adherence but also significantly reduces risks linked to compliance violations, which have seen notable increases in costs due to lost business and response efforts.
Interacting with Oversight Agencies fosters essential connections with governing authorities. Such relationships promote open dialogue and provide insights into upcoming changes. Successful engagement in regulatory affairs in pharmaceuticals can lead to more efficient approval processes and a deeper understanding of compliance expectations, as evidenced by entities that have adeptly navigated complex compliance environments.
Participating in industry associations and forums allows professionals to exchange knowledge and gain insights from peers regarding best practices and compliance developments. Notably, data indicates that 34% of organizations outsource compliance tasks, underscoring the significance of collaboration and shared knowledge in overcoming legal challenges.
Adjusting to Global Harmonization is increasingly important. As oversight entities pursue harmonization, organizations engaged in regulatory affairs in pharmaceuticals should align their procedures with international standards to facilitate access to global markets. This synchronization streamlines compliance and enhances the ability to respond to diverse legal requirements across regions, ultimately supporting faster product launches and improved patient access to innovative therapies.

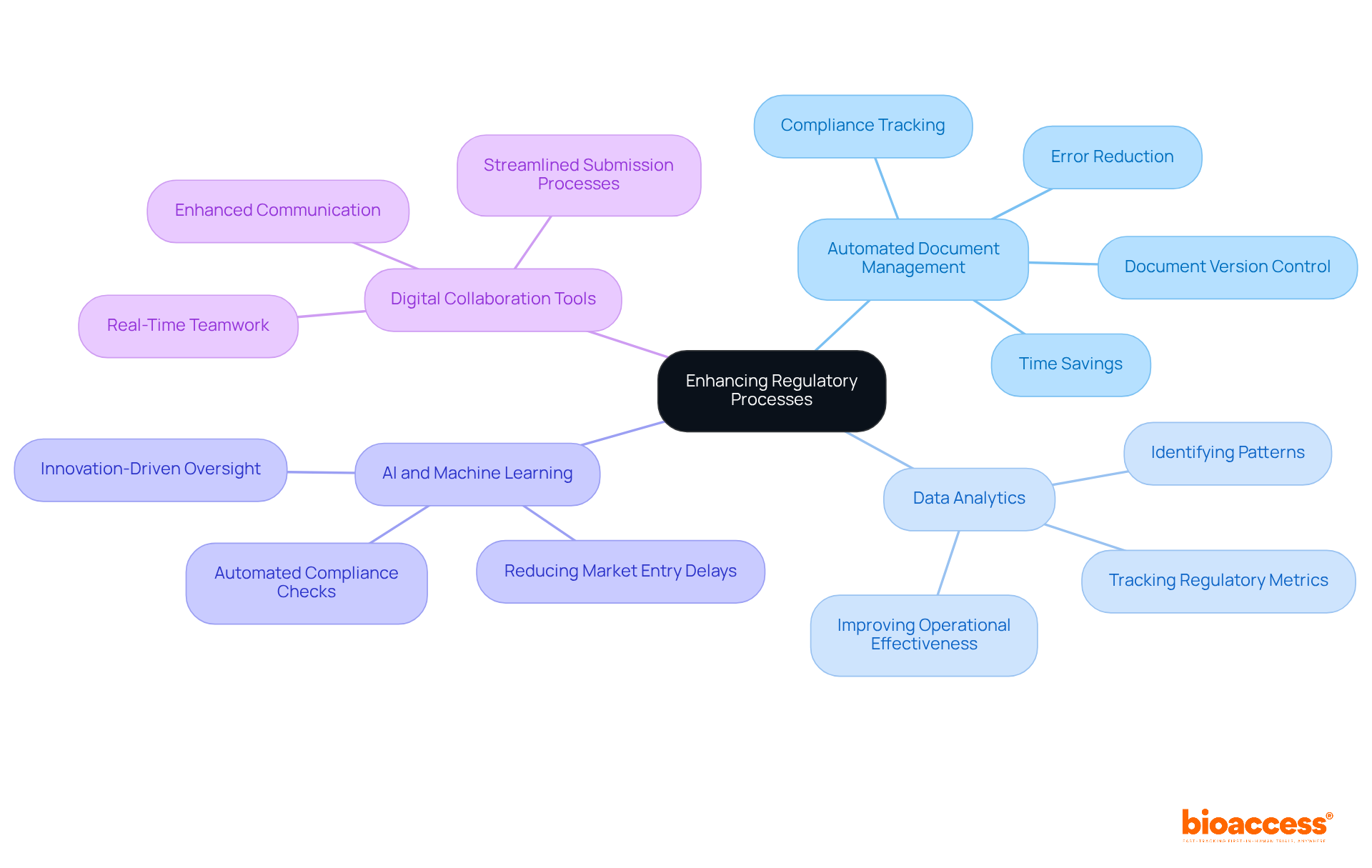
Organizations can significantly enhance their regulatory affairs in pharmaceuticals by leveraging advanced technology in several impactful ways.
Automated Document Management: Implementing document management systems (DMS) automates the storage, retrieval, and tracking of compliance documents. This not only guarantees adherence but also minimizes manual mistakes. Automated workflows for document creation, review, approval, and distribution can decrease time spent on these tasks by up to 50%. Systems like smartDOC centralize submission-related documents, ensuring that the latest versions are always accessible and compliant with standards.
Data Analytics: Employing data analytics tools enables organizations to efficiently track regulatory metrics and recognize patterns that guide decision-making and enhancements. By examining oversight data, businesses can acquire strategic knowledge that improves operational effectiveness and adherence stance.
AI and Machine Learning: Investigating AI-powered solutions for submissions and oversight can simplify processes and improve accuracy. Automated systems equipped with built-in compliance checks help organizations stay aligned with changing regulations, reducing the risk of market entry delays by as much as 35%. This shift towards automation transforms oversight functions from compliance-driven to innovation-led, allowing teams to focus on strategic initiatives rather than routine paperwork.
Digital Collaboration Tools: Adopting digital collaboration platforms facilitates seamless communication and teamwork among cross-functional teams. Collaboration tools in automated document management systems enable real-time teamwork across departments, ensuring alignment on compliance requirements and timelines. This enhances overall efficiency and decreases the chances of miscommunication. Real-time collaboration features in automated systems allow teams to work together effectively, regardless of geographical locations, thereby expediting the submission process.
By integrating these technologies, organizations can not only improve their regulatory affairs in pharmaceuticals but also foster a culture of innovation and agility in their operations.
Regulatory affairs in pharmaceuticals are essential for ensuring that products meet rigorous safety and efficacy standards while navigating complex compliance requirements. This field acts as a vital link between the pharmaceutical industry and regulatory bodies, highlighting the necessity of effective communication, thorough documentation, and proactive engagement with oversight agencies. As the market for regulatory compliance expands, mastering these affairs becomes increasingly critical.
Key insights underscore the need for adopting best practices that enhance compliance and operational efficiency. Organizations must:
Additionally, staying vigilant in monitoring regulatory updates and leveraging technology to streamline processes is crucial for adapting to the ever-evolving regulatory landscape.
In light of these findings, organizations in the pharmaceutical sector must embrace a forward-thinking approach to regulatory affairs. By prioritizing compliance and integrating innovative technologies, companies can safeguard their operations while driving the successful development and timely market access of new therapies. Proactively adapting to regulatory changes will ultimately enhance patient access to essential medications and cultivate a culture of continuous improvement within the industry.
What is regulatory affairs in pharmaceuticals?
Regulatory affairs in pharmaceuticals encompasses the processes and activities that ensure compliance with regulations governing the development, testing, and marketing of pharmaceutical products. It serves as a vital link between the pharmaceutical industry and regulatory bodies, ensuring that products are safe, effective, and of high quality.
What are the main responsibilities of professionals in regulatory affairs?
Professionals in regulatory affairs are responsible for preparing and submitting documentation to governing agencies, monitoring compliance with regulations, and facilitating communication among stakeholders.
Why is regulatory affairs important in drug development?
Regulatory affairs are critical in navigating the complex landscape of drug development, as they ensure compliance and foster innovation, ultimately driving the success of drug development initiatives.
What is the projected growth of the global pharmaceutical compliance market?
The global pharmaceutical compliance market is projected to reach USD 20.56 billion by 2033, expanding at a compound annual growth rate (CAGR) of 8.10% from 2025 to 2033.
What strategies are effective for compliance within pharmaceutical firms?
Effective compliance strategies include proactive engagement with regulatory agencies, a comprehensive understanding of evolving guidelines, and the integration of advanced technologies to streamline regulatory affairs.
How do regulatory affairs contribute to innovation in pharmaceuticals?
Efficient regulatory affairs extend beyond merely fulfilling requirements; they also foster innovation and ensure timely market access for new therapies as the regulatory landscape evolves.