


This article delineates the fundamental steps and skills necessary for mastering regulatory medical writing, highlighting the critical importance of comprehending regulatory guidelines and excelling in effective communication. It does so by presenting a structured approach to document creation, elucidating the roles and responsibilities of medical writers, and offering strategies to navigate common challenges encountered in the regulatory process. This comprehensive framework ultimately facilitates successful submissions and approvals.
Regulatory medical writing stands as a pivotal element in the journey of bringing medical products to market, serving as the backbone of compliance documentation that facilitates approvals from regulatory bodies. This guide explores the essential skills and structured processes necessary to master this intricate field, offering readers valuable insights into effective writing practices and the roles of medical writers. As the landscape of regulatory requirements continues to evolve, aspiring writers must consider how to navigate these complexities and ensure their submissions distinguish themselves amidst stringent scrutiny.
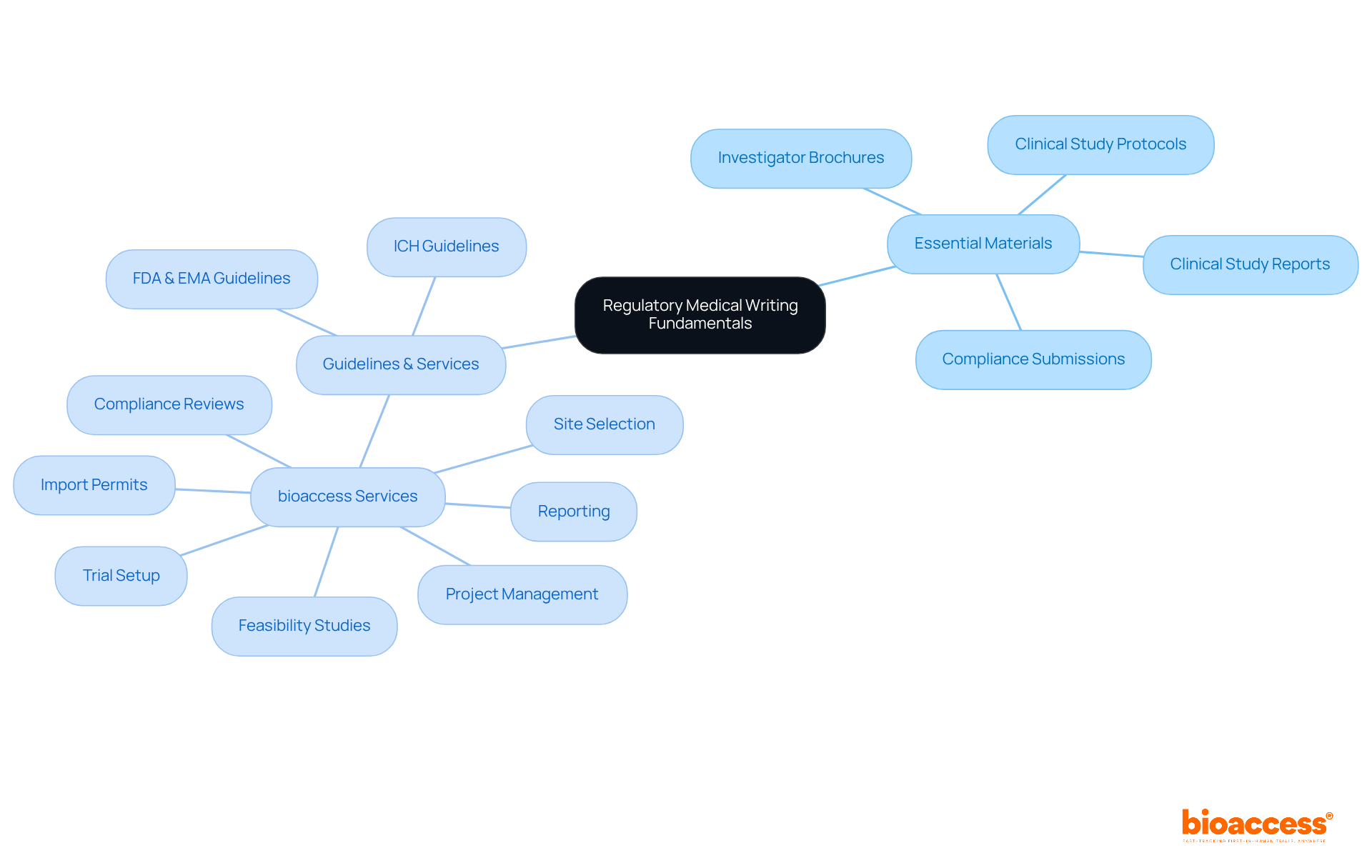
Regulatory medical writing plays a pivotal role in facilitating the approval process for medical products. This encompasses the production of essential materials such as:
A thorough understanding of the guidelines established by regulatory bodies like the FDA and EMA is paramount for effective regulatory medical writing. Familiarity with the International Conference on Harmonisation (ICH) guidelines is also crucial for regulatory medical writing, as they provide a structured approach to creating these materials.
Moreover, clarity, accuracy, and adherence to formatting standards cannot be overstated; these elements are critical for successful submissions and approvals. At bioaccess, we stand at the forefront of trial management services, offering comprehensive solutions that include:
Our expertise extends to reviewing and providing feedback on study materials, ensuring compliance with country-specific requirements, thereby facilitating a smoother approval process.
In the evolving Medtech landscape, collaboration is essential. By partnering with bioaccess, you can navigate the complexities of clinical research with confidence. We invite you to explore how our services can address your key challenges and streamline your path to success.
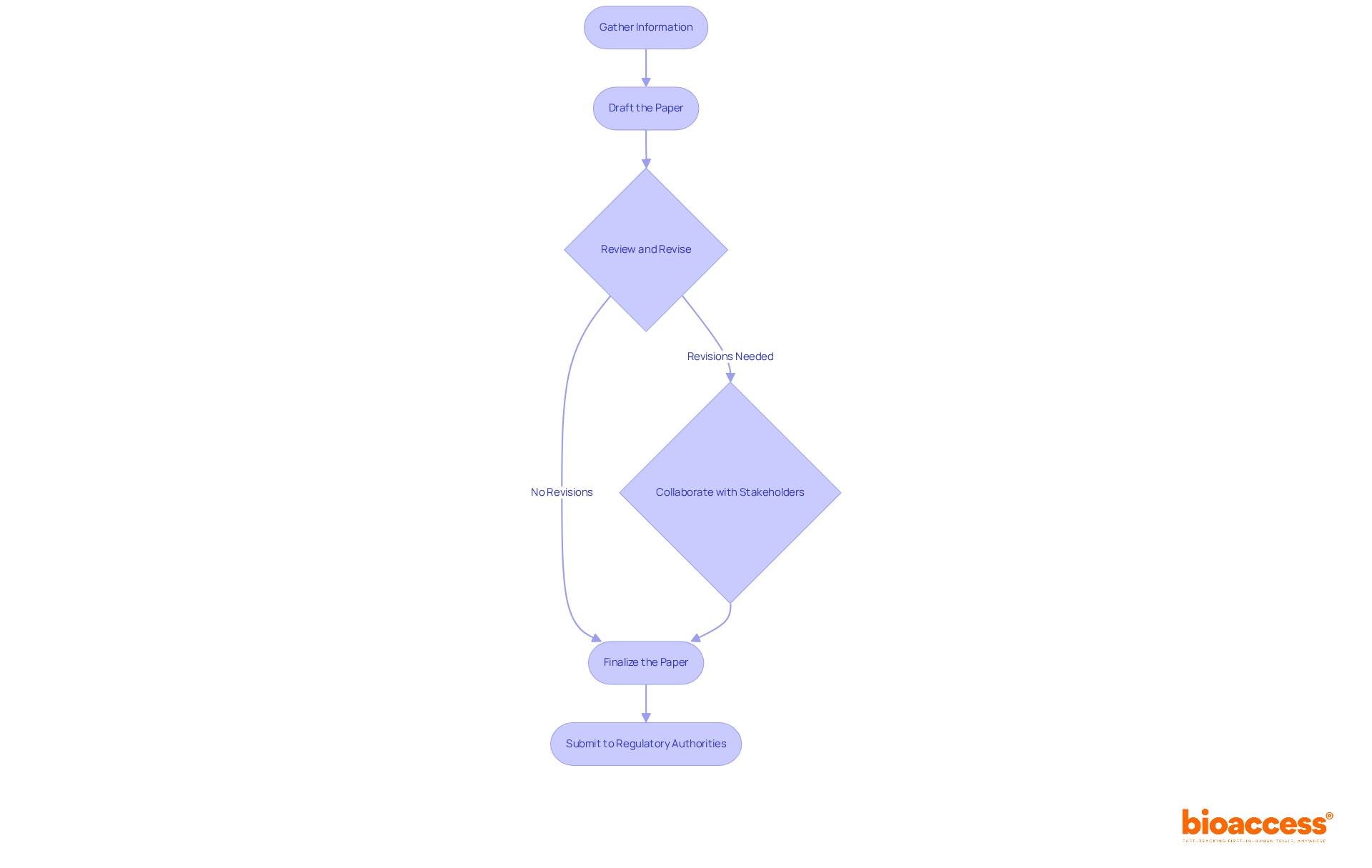
Medical writers play a pivotal role within the research environment, particularly in regulatory medical writing, which involves the composition, evaluation, and completion of essential official documents. Their responsibilities in regulatory medical writing encompass:
This includes evaluating and commenting on research materials to satisfy national requirements. Effective communication with various stakeholders—including oversight bodies, healthcare teams, and project managers—is vital for maintaining the integrity and clarity of documentation.
At bioaccess, our comprehensive trial management services include:
This holistic approach ensures that compliance writers receive the necessary support and resources to excel in their roles. Collaboration between compliance authors and clinical teams is essential, as it facilitates a seamless exchange of information and enhances the quality of submissions. On average, compliance writers dedicate significant time to document preparation, often spending over 40% of their work hours on this task. This underscores the necessity for robust project management skills and a deep understanding of timelines in regulatory medical writing, as official submissions are frequently bound by strict deadlines that, if missed, can lead to delays in product approval.
The skills required for regulatory medical writing in compliance extend beyond mere technical writing; they include:
As emphasized by industry leaders, effective communication transcends the mere delivery of information; it is about ensuring that the message resonates with the audience. This is particularly critical in regulatory medical writing related to compliance documentation, where clarity and precision significantly impact the approval process and, ultimately, the success of a product in the marketplace. With insights from specialists such as Ana Criado, Director of Compliance Affairs, and Katherine Ruiz, an authority in Compliance Affairs for Medical Devices and In Vitro Diagnostics, bioaccess is exceptionally equipped to navigate the complexities of medical documentation.

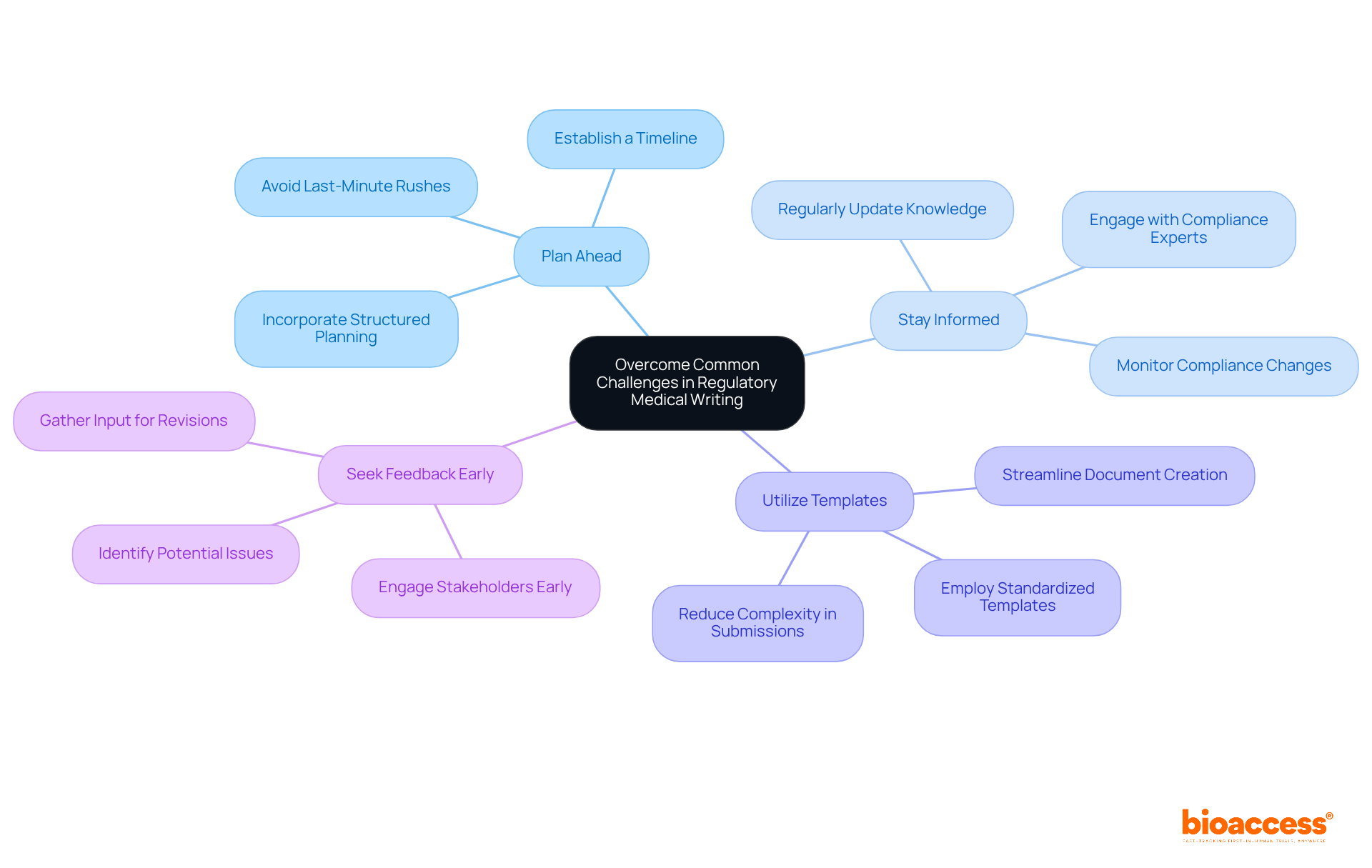
Common challenges in regulatory medical writing encompass tight deadlines, complex regulatory requirements, and the necessity for precise data interpretation. To effectively address these challenges, consider the following strategies:
Plan Ahead: Establish a timeline for each document and adhere to it, thereby avoiding last-minute rushes. Drawing from the expertise of Ana Criado, Director of Regulatory Affairs with extensive experience at INVIMA and a background in biomedical engineering, it is essential to incorporate structured planning into your writing process.
Stay Informed: Regularly update your knowledge of compliance changes and guidelines to ensure adherence. With professionals like Katherine Ruiz, who focuses on compliance matters for medical devices and in vitro diagnostics, remaining informed becomes more manageable.
Utilize Templates: Employ standardized templates for common documents to streamline the creation process. This practice can significantly reduce the complexity often associated with compliance submissions.
Seek Feedback Early: Engage stakeholders early in the writing process to identify potential issues and gather input, which can save time during the revision phase. Leveraging insights from experienced consultants like Ana, who has collaborated with global companies, can enhance the quality of feedback received.
Tight deadlines can adversely affect the quality of compliance submissions, leading to increased scrutiny and potential delays in approval. By implementing these strategies, regulatory medical writing can enhance the efficiency and quality of submissions, ultimately facilitating smoother regulatory processes.
Mastering regulatory medical writing is essential for ensuring the successful approval of medical products. This discipline not only requires a thorough understanding of regulatory guidelines but also emphasizes the importance of clarity, accuracy, and collaboration among stakeholders. By honing these skills and following a structured approach, medical writers can significantly impact the efficiency of the regulatory process.
The article outlines the fundamental aspects of regulatory medical writing, including the roles and responsibilities of medical writers, the step-by-step process for creating essential documents, and strategies for overcoming common challenges. Key insights, such as the necessity for meticulous planning and staying informed about compliance changes, serve as valuable guidance for aspiring regulatory writers. Additionally, the support offered by organizations like bioaccess underscores the importance of collaboration in navigating the complexities of clinical research.
Ultimately, the significance of regulatory medical writing cannot be overstated. It is a critical component in the journey of medical products from conception to market. By mastering the principles and practices outlined in this guide, individuals can contribute to a more streamlined approval process, ensuring that innovative medical solutions reach those in need. Embracing this knowledge and fostering collaborative relationships will pave the way for success in the ever-evolving landscape of medical research and regulatory affairs.
What is the role of regulatory medical writing?
Regulatory medical writing plays a crucial role in facilitating the approval process for medical products by producing essential materials such as clinical study protocols, investigator brochures, clinical study reports, and compliance submissions.
Why is it important to understand regulatory guidelines?
A thorough understanding of guidelines established by regulatory bodies like the FDA and EMA is essential for effective regulatory medical writing, as it ensures compliance and increases the likelihood of successful submissions and approvals.
What are the key guidelines that regulatory medical writers should be familiar with?
Regulatory medical writers should be familiar with the International Conference on Harmonisation (ICH) guidelines, which provide a structured approach to creating regulatory documents.
What are the critical elements of successful regulatory submissions?
Clarity, accuracy, and adherence to formatting standards are critical elements for successful regulatory submissions and approvals.
What services does bioaccess offer in relation to regulatory medical writing?
Bioaccess offers comprehensive trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
How does bioaccess assist with compliance?
Bioaccess reviews and provides feedback on study materials to ensure compliance with country-specific requirements, facilitating a smoother approval process.
Why is collaboration important in the Medtech landscape?
Collaboration is essential in the evolving Medtech landscape to navigate the complexities of clinical research effectively and to address key challenges in the approval process.