


The article outlines a five-step process for mastering regulatory project management in Mexico's Medtech sector, emphasizing the critical steps necessary to navigate compliance with COFEPRIS and related regulations. Understanding device classification is paramount, as it lays the groundwork for effective documentation preparation. Engaging local experts further enhances compliance management, ensuring successful entry and operation within Mexico's medical device market. This market is poised for significant growth, underscoring the importance of ongoing compliance management to capitalize on emerging opportunities.
Navigating the complex landscape of medical technology regulations in Mexico presents a formidable challenge for manufacturers and stakeholders alike. With the healthcare market projected to soar to $5.5 billion, grasping the intricacies of compliance is not merely advantageous; it is essential for successful market entry. This guide delineates a strategic five-step approach to mastering regulatory project management within Mexico's Medtech sector, addressing critical challenges and opportunities that lie ahead.
How can manufacturers effectively streamline their compliance processes while adapting to evolving regulations and ensuring timely market access?
To effectively navigate the Medtech compliance framework in Mexico, understanding the primary governing entity—the Federal Commission for Protection against Sanitary Risks (COFEPRIS)—is essential. COFEPRIS oversees the authorization and validation of medical equipment, ensuring compliance with the General Health Law.
It is imperative to familiarize yourself with the classification of medical devices according to risk levels, as this classification dictates the regulatory pathway you must pursue. Additionally, staying informed about recent changes, such as the 2025 Acuerdo, is crucial. This new regulation introduces requirements for low-risk products that now necessitate sanitary approval. Devices previously exempt from documentation must now comply with these updated regulations, with a grace period extending until 2029. Understanding these components will provide a solid foundation for your compliance project management efforts in Mexico.
Moreover, the anticipated evaluation period for health equipment registration in 2025 is projected to be between 3 to 8 months. Engaging an Authorized Third Party can significantly expedite this process, reducing the timeframe to as little as 1 to 3 months. Given that nearly 90% of healthcare devices sold in Mexico are imports, securing a local compliance representative is vital for ensuring adherence and facilitating successful market entry.
By remaining abreast of these legislative changes, manufacturers can strategically position themselves within Mexico's rapidly growing healthcare market, which is projected to reach a value of $5.5 billion.
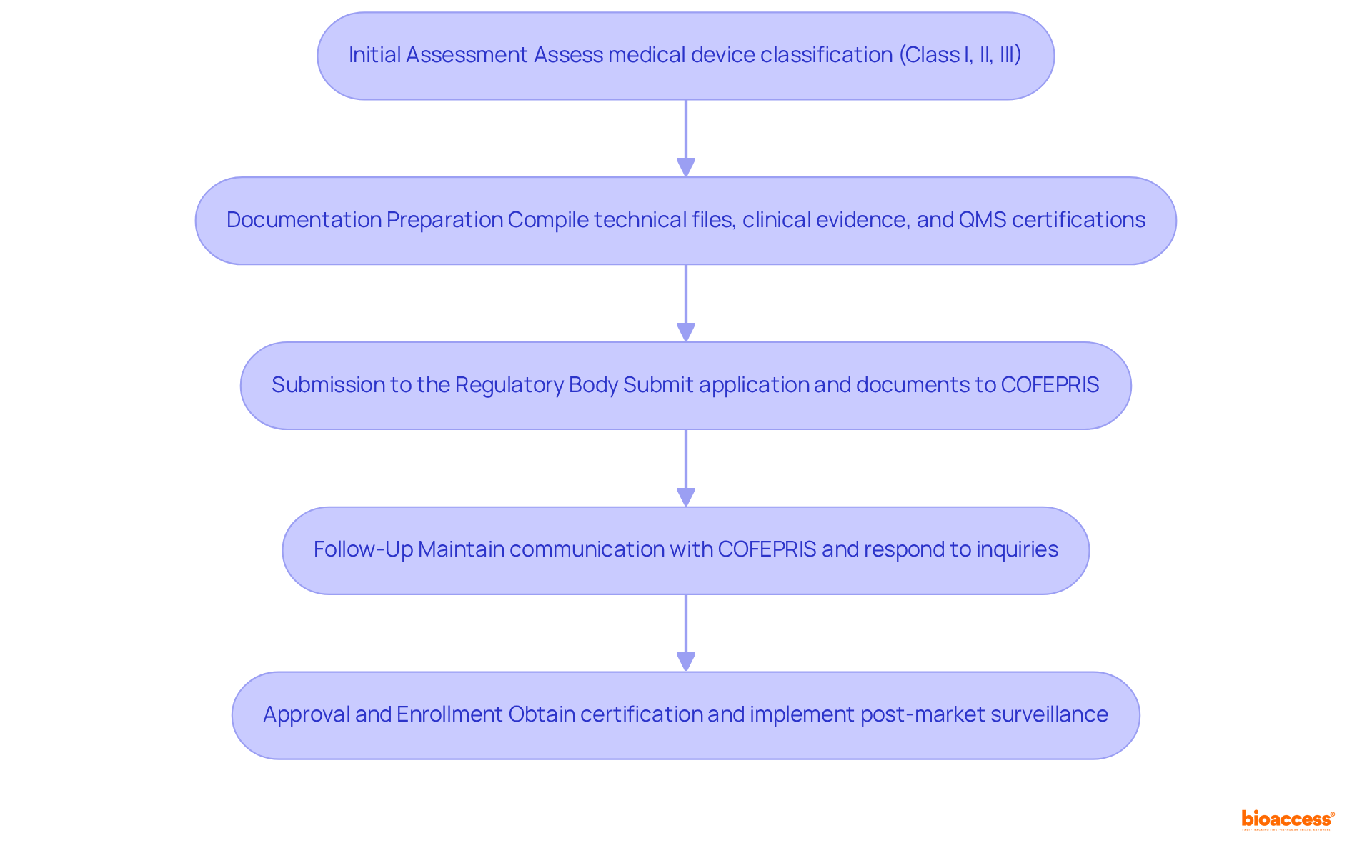
Initial Assessment: Begin by assessing your medical device's classification, which is essential for identifying the specific documentation needed for regulatory registration. Understanding the classification criteria—Class I (low risk), Class II (medium risk), and Class III (high risk)—is crucial for regulatory project management in Mexico's medtech landscape.
Documentation Preparation: Compile all necessary documents, including technical files, clinical evidence, and quality management system certifications. Ensure that all documentation is translated into Spanish, as this is a mandatory requirement for submission. If documents are not originally in Spanish, they must be officially certified. A complete technical dossier should include a detailed technical description, international certifications, and a user manual in Spanish.
Submission to the Regulatory Body: Submit your application along with the required documentation to the agency. The usual evaluation period for applications generally spans from 3 to 8 months, but the approval process may require as little as 6 months in the most favorable circumstances. Utilizing an Authorized Third Party can expedite this process to as little as 1 to 3 months, as TPRs can reduce approval timelines by up to 30% compared to traditional reviews. Be prepared for possible inquiries or demands for additional information during the evaluation process, as gaps in documentation can considerably postpone approval, potentially extending the process to 26 months.
Follow-Up: Maintain proactive communication with the regulatory agency to monitor the status of your application. Quickly responding to any questions or requests for clarification can help prevent unnecessary delays in the enrollment process. It is also advisable to collaborate with experts in regulatory project management Mexico medtech, as their expertise can effectively address potential challenges in navigating regulatory frameworks.
Approval and Enrollment: Upon receiving approval, ensure that you obtain your certification. This document is essential for promoting your product in Mexico, as the registration is valid for five years and must be renewed with the necessary paperwork submitted 150 days before expiration. Additionally, manufacturers must implement post-market surveillance (PMS) activities to monitor product performance and ensure ongoing safety and effectiveness.
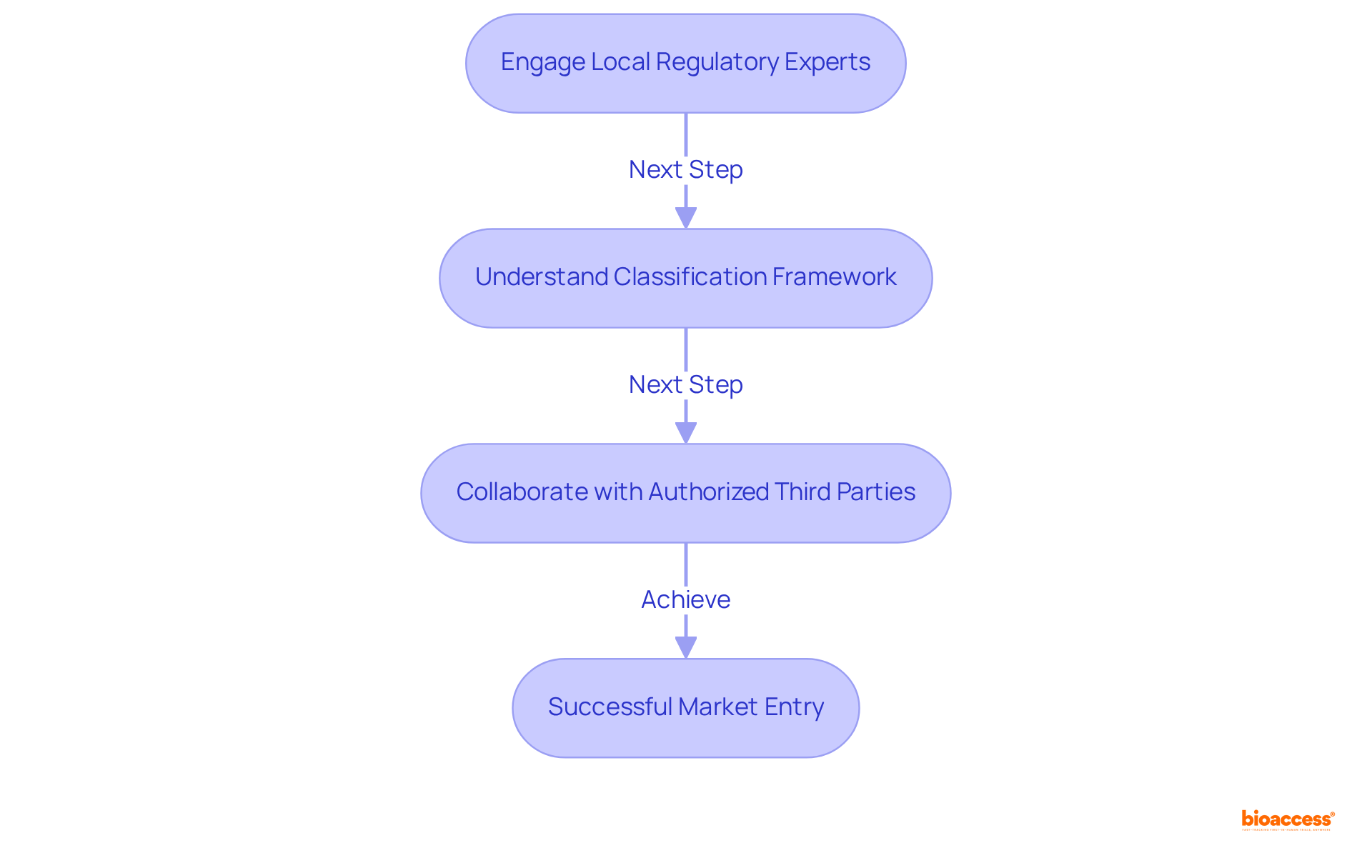
To navigate the compliance landscape in Mexico effectively, it is crucial to engage local consultants or specialists who possess in-depth knowledge of regulatory project management Mexico medtech and the specific requirements for medical devices. These professionals offer invaluable insights into the regulatory process, assist in documentation preparation, and help you sidestep common pitfalls that could result in fines, legal actions, or even business closures due to non-compliance.
As compliance expert Katherine Ruiz aptly notes, "A comprehensive understanding of the classification framework is vital for achieving successful market entry." Moreover, employing Authorized Third Parties (TPRs) can significantly reduce review times, thereby accelerating the approval process.
Establishing a robust collaboration with regional specialists not only facilitates communication with the regulatory body but also supports effective regulatory project management in Mexico medtech, enhancing your chances of a successful and prompt approval, ultimately ensuring a smoother market entry for your medical products.
After successfully registering your medical apparatus with the regulatory authority, it is essential to apply regulatory project management Mexico medtech for managing ongoing compliance and reporting obligations. This includes:
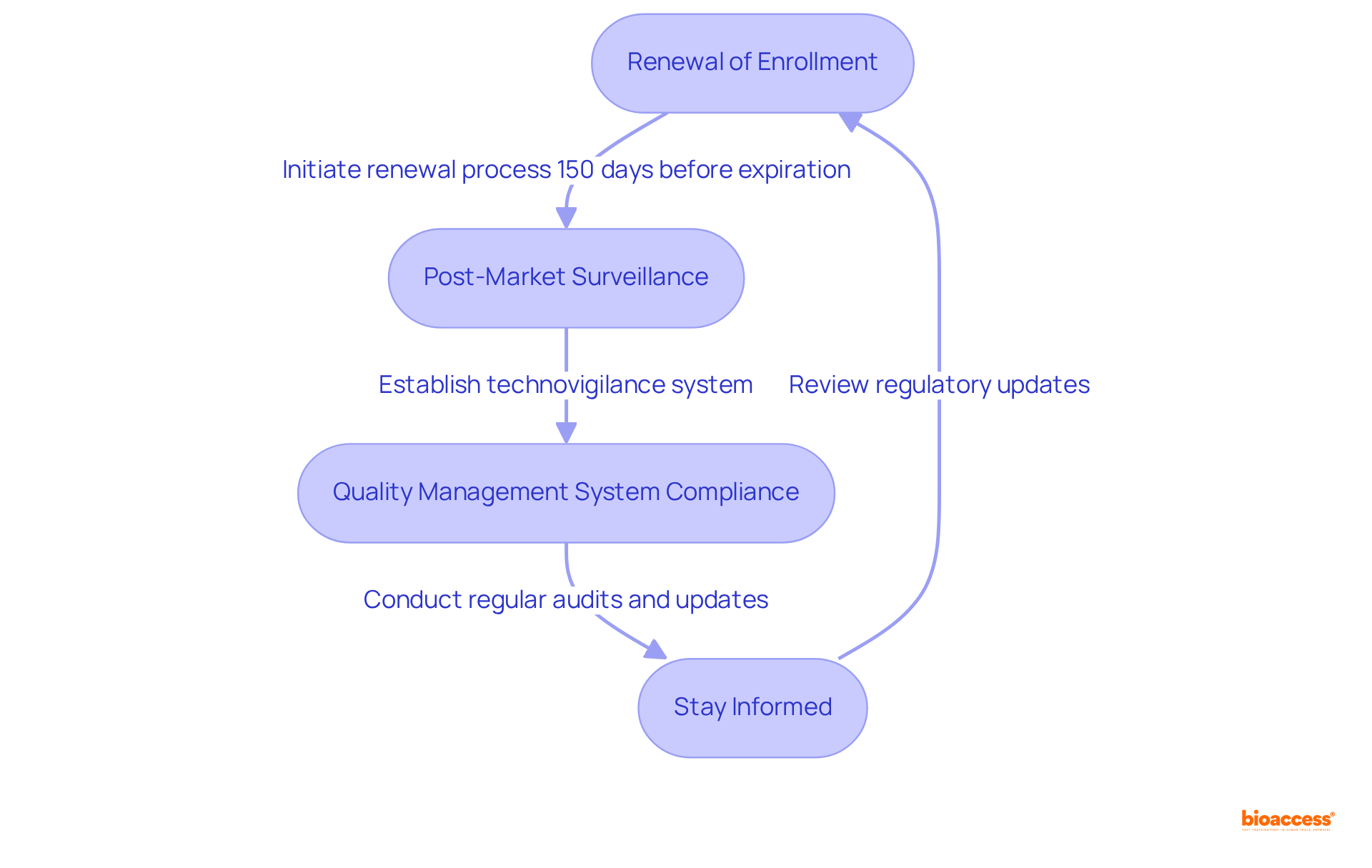
Renewal of Enrollment: Medical equipment approvals in Mexico are valid for five years. Initiate the renewal process at least 150 days before expiration to prevent lapses in compliance, as timely submissions are essential for maintaining market access. In the realm of regulatory project management Mexico medtech, the initial extension for medical product registration requires documentation such as a Technovigilance Report, which is essential for compliance.
Post-Market Surveillance: Establish a robust system for monitoring your device's performance in the market. This involves complying with technovigilance duties by notifying the regulatory authority of any adverse events, ensuring that safety and effectiveness are consistently assessed. Under NOM-240, establishing a technovigilance system is a specific requirement that must be met.
Quality Management System Compliance: Maintain compliance with Good Manufacturing Practices (GMP) and other relevant standards. To ensure effective regulatory project management in Mexico medtech, regular audits and updates to your quality management system are necessary to uphold compliance and address any regulatory changes, ensuring that your practices remain aligned with evolving standards.
Stay Informed: Remain vigilant about any updates in regulations or guidelines from COFEPRIS. Regularly review and adapt your compliance processes within the framework of regulatory project management in Mexico medtech to align with new requirements, ensuring ongoing adherence and minimizing risks associated with non-compliance. With Mexico's Medical Devices market projected to reach US$8.09 billion in 2024, staying informed is essential in this rapidly evolving landscape.
Navigating the complex regulatory landscape of Mexico's Medtech sector necessitates a profound understanding of the governing framework, particularly the pivotal role of COFEPRIS and the recent regulatory changes that influence market entry. By mastering the five-step regulatory project management process outlined, manufacturers can strategically position their medical devices for success in this rapidly expanding market.
This article underscores the critical importance of:
Engaging local regulatory experts and utilizing Authorized Third Parties can significantly streamline the approval process, ensuring compliance and minimizing potential delays. Furthermore, ongoing post-registration compliance, including timely renewals and robust post-market surveillance, is essential for sustaining market access and guaranteeing the safety and effectiveness of medical devices.
In conclusion, the importance of mastering regulatory project management in Mexico's Medtech landscape cannot be overstated. With the market projected to experience substantial growth, manufacturers are urged to:
By doing so, they not only enhance their prospects for successful market entry but also contribute to the overall safety and advancement of healthcare within Mexico. Embracing these strategies will pave the way for sustained growth and innovation in the Medtech sector.
What is the primary governing entity for Medtech in Mexico?
The primary governing entity for Medtech in Mexico is the Federal Commission for Protection against Sanitary Risks (COFEPRIS).
What does COFEPRIS oversee?
COFEPRIS oversees the authorization and validation of medical equipment, ensuring compliance with the General Health Law.
Why is it important to understand the classification of medical devices?
Understanding the classification of medical devices according to risk levels is important because it dictates the regulatory pathway that must be pursued for compliance.
What is the significance of the 2025 Acuerdo?
The 2025 Acuerdo introduces new requirements for low-risk products that now necessitate sanitary approval, affecting devices that were previously exempt from documentation.
What is the grace period for compliance with the new regulations introduced by the 2025 Acuerdo?
The grace period for compliance with the new regulations extends until 2029.
What is the projected evaluation period for health equipment registration in 2025?
The projected evaluation period for health equipment registration in 2025 is between 3 to 8 months.
How can engaging an Authorized Third Party benefit the registration process?
Engaging an Authorized Third Party can significantly expedite the registration process, reducing the timeframe to as little as 1 to 3 months.
What percentage of healthcare devices sold in Mexico are imports?
Nearly 90% of healthcare devices sold in Mexico are imports.
Why is securing a local compliance representative important?
Securing a local compliance representative is vital for ensuring adherence to regulations and facilitating successful market entry in Mexico.
What is the projected value of Mexico's healthcare market?
Mexico's healthcare market is projected to reach a value of $5.5 billion.