


The article centers on mastering risk and regulatory consulting, crucial for achieving success in clinical research. It underscores the significance of:
This is illustrated by examples demonstrating how:
Navigating the intricate landscape of clinical research necessitates a profound understanding of regulatory frameworks and effective risk management strategies. Professionals in this field can gain invaluable insights into optimizing their research processes, ensuring compliance, and ultimately enhancing study outcomes. Yet, a critical challenge persists: how can organizations effectively integrate proactive risk assessment and foster collaboration among diverse stakeholders to mitigate potential pitfalls? Exploring these essential elements can unlock the key to successful clinical research endeavors.
To master the regulatory landscape, clinical research professionals must familiarize themselves with the guidelines established by various regulatory bodies, including the FDA, EMA, and ICH. This involves a thorough understanding of Good Clinical Practice (GCP) guidelines, which outline the ethical and scientific quality standards essential for designing, conducting, recording, and reporting studies.
Furthermore, it is crucial for professionals to stay informed about local regulations in regions where research is conducted, particularly in:
Engaging with risk & regulatory consulting and participating in workshops can significantly enhance understanding and compliance. A recent case analysis illustrates this point: a comprehensive grasp of local regulations led to a remarkable 30% reduction in approval times for a biopharma client conducting tests in Brazil. By actively addressing regulatory obligations, organizations can mitigate uncertainties and bolster the likelihood of favorable research outcomes.

Applying proactive threat evaluation strategies is crucial for identifying potential hazards at the onset of a clinical experiment and monitoring them throughout the investigation. Techniques such as Failure Mode and Effects Analysis (FMEA) enable systematic evaluation of processes, pinpointing where failures may arise. Moreover, risk & regulatory consulting highlights that risk-based monitoring approaches empower teams to allocate resources effectively toward higher-risk areas, ensuring the precise collection of critical data. A recent study illustrates this point, showing that a proactive management strategy led to a 40% reduction in protocol deviations within a multi-site study. To foster a culture of continuous improvement and vigilance within the research team, it is essential to:

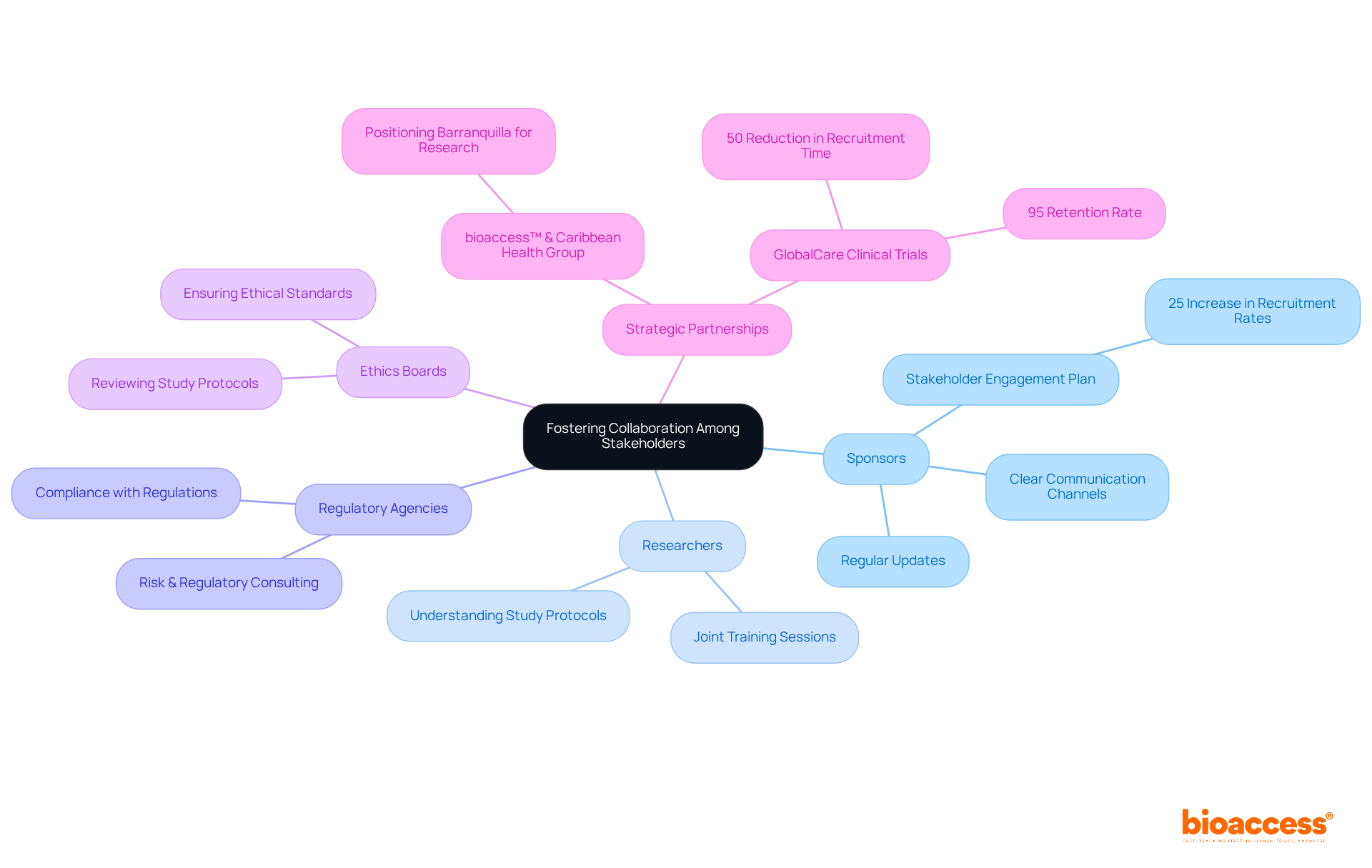
Encouraging cooperation among stakeholders—including sponsors, researchers, regulatory agencies, and ethics boards—is crucial for the success of medical studies. Establishing clear communication channels and providing regular updates ensures that all parties remain informed and engaged.
For instance, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading location for research studies in Latin America, backed by Colombia's Minister of Health. This initiative exemplifies how strategic partnerships can enhance research environments.
Moreover, GlobalCare Clinical Trials, in collaboration with bioaccess™, achieved over a 50% reduction in recruitment time while maintaining a 95% retention rate, illustrating the effectiveness of collaborative efforts.
Utilizing collaborative platforms and tools facilitates real-time communication and document sharing, thereby minimizing misunderstandings and delays. A biopharma company that implemented a stakeholder engagement plan reported a 25% increase in recruitment rates due to improved communication with site staff.
Furthermore, organizing joint training sessions enhances understanding of study protocols and regulatory requirements, which is vital in the context of risk & regulatory consulting, further strengthening collaboration.
By prioritizing stakeholder engagement, organizations can foster a supportive environment that propels clinical research success.
Mastering risk and regulatory consulting is pivotal for achieving success in clinical research. Understanding the intricate regulatory frameworks established by organizations such as the FDA, EMA, and ICH is essential for professionals aiming to navigate the complexities of clinical trials effectively. This knowledge not only enhances compliance but also significantly impacts the efficiency and outcomes of research endeavors.
The article highlights several key strategies for optimizing clinical research processes. These strategies include:
By employing methods such as Failure Mode and Effects Analysis and establishing clear communication channels among all parties involved, organizations can mitigate risks, reduce approval times, and enhance recruitment rates. Documented successes in various case studies underscore the tangible benefits of these best practices.
Ultimately, the significance of effective risk and regulatory consulting cannot be overstated. As the landscape of clinical research continues to evolve, embracing these best practices will be crucial for organizations seeking to improve their operational efficiency and research outcomes. Stakeholders are encouraged to prioritize collaboration and proactive risk management strategies to create a supportive environment that drives clinical research success. The future of clinical trials hinges on the ability to adapt and innovate within the regulatory framework, ensuring that research not only meets compliance standards but also achieves its ultimate goals.
What is the importance of understanding regulatory frameworks in clinical research?
Understanding regulatory frameworks is essential for clinical research professionals to comply with guidelines established by regulatory bodies like the FDA, EMA, and ICH, ensuring ethical and scientific quality in studies.
What are Good Clinical Practice (GCP) guidelines?
Good Clinical Practice (GCP) guidelines outline the ethical and scientific quality standards necessary for designing, conducting, recording, and reporting clinical research studies.
Which regions should professionals be particularly aware of regarding local regulations?
Professionals should be aware of local regulations in regions such as Latin America, the Balkans, and Australia.
How can engaging with risk and regulatory consulting benefit clinical research professionals?
Engaging with risk and regulatory consulting can enhance understanding and compliance with regulatory requirements, improving the overall quality and efficiency of clinical research.
What impact can a thorough understanding of local regulations have on research approval times?
A comprehensive understanding of local regulations can lead to significant reductions in approval times, as demonstrated by a case analysis where a biopharma client in Brazil experienced a 30% reduction in approval times.
How can organizations mitigate uncertainties in clinical research?
Organizations can mitigate uncertainties by actively addressing regulatory obligations, which increases the likelihood of favorable research outcomes.