


The article underscores the critical importance of mastering statistical concepts in clinical trials for effective research management. It asserts that a deep understanding of key statistical methods—such as randomization, control groups, and the interpretation of p-values and confidence intervals—is essential for researchers. This knowledge enables them to draw valid conclusions and ensures the reliability of their findings in clinical research.
Understanding the nuances of statistics in clinical trials is essential for effective research management, as it serves as the backbone of credible scientific inquiry. Mastering key statistical concepts enables researchers not only to interpret complex data but also to navigate regulatory requirements and enhance the reliability of their findings.
However, the landscape of clinical research presents significant challenges, including common misinterpretations and biases that can skew results. How can researchers equip themselves to overcome these obstacles and leverage statistical analysis to drive impactful clinical outcomes? This inquiry is crucial as it sets the stage for addressing the complexities inherent in clinical research.
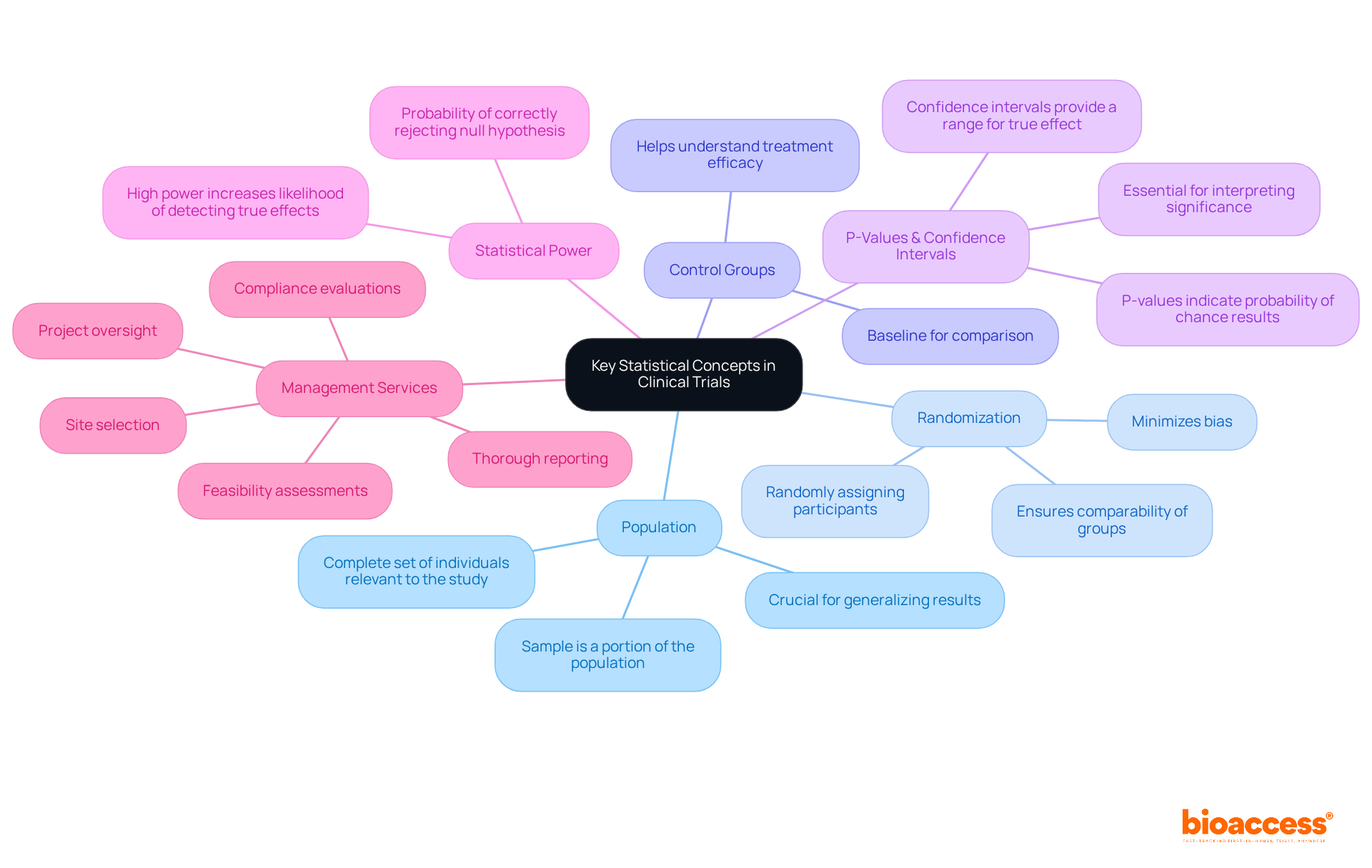
In research studies, several essential quantitative concepts, particularly statistics in clinical trials, are crucial for efficient research oversight. These include:
Alongside these quantitative concepts, efficient management services for studies, like those provided by bioaccess, encompass feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and thorough reporting. These services not only support compliance with INVIMA regulatory functions but also enhance the comprehension and application of quantitative concepts. This allows researchers to better navigate the intricacies of data evaluation in medical trials.
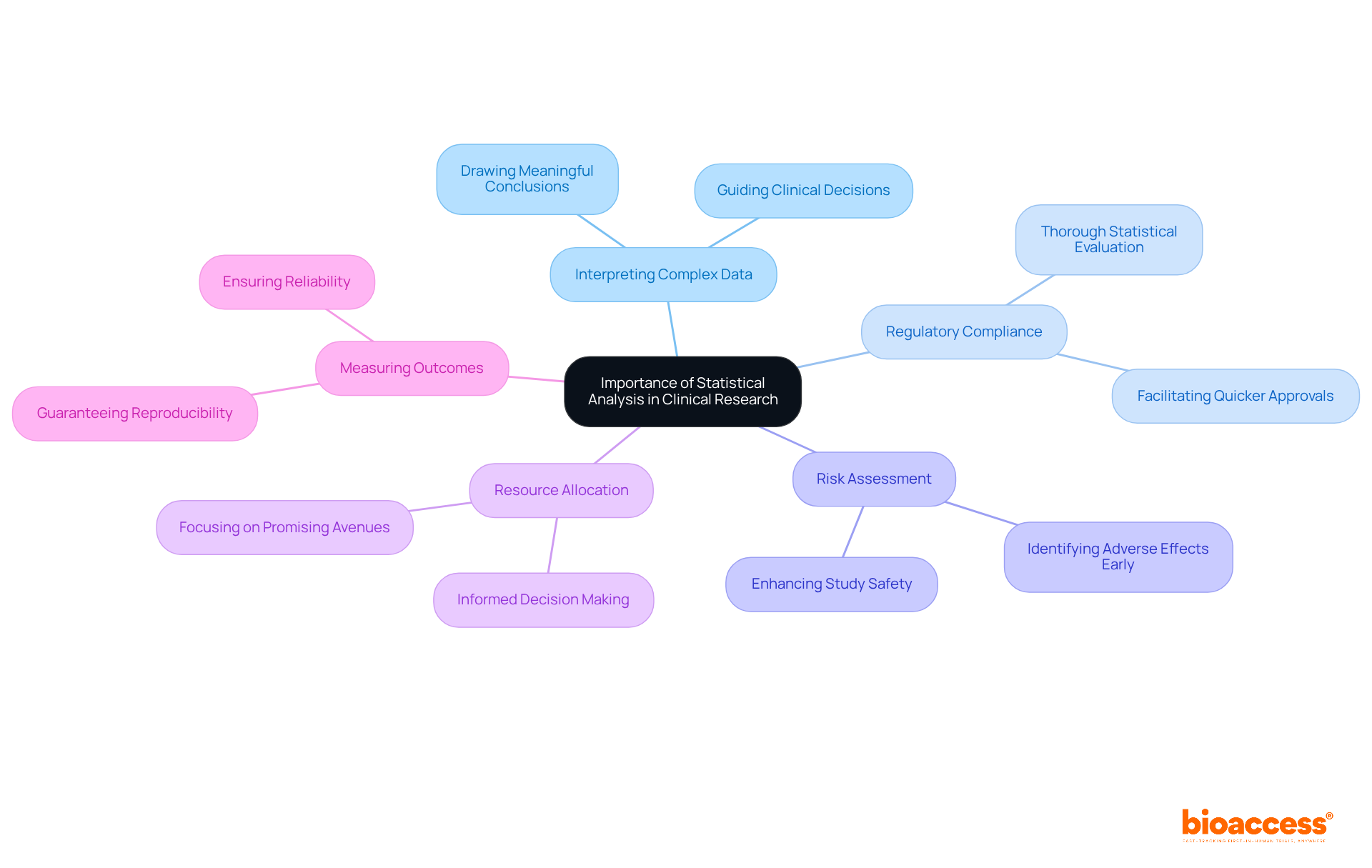
The use of statistics in clinical trials is essential in clinical research for several compelling reasons.
In conclusion, emphasizing the importance of data examination empowers researchers to recognize its vital contribution to enhancing the reliability and impact of their studies.
In clinical trials, statistics in clinical trials play a pivotal role through several statistical methods, each serving distinct purposes that enhance the research process.
Descriptive Statistics summarize the basic features of the data, offering simple summaries about the sample and measures. These include essential metrics such as mean, median, mode, and standard deviation.
Meanwhile, Inferential Statistics enable researchers to make predictions or inferences about a population based on a sample, employing techniques like t-tests, chi-square tests, and ANOVA to draw meaningful conclusions.
Regression Analysis is instrumental in understanding the relationship between variables, helping to identify factors that influence treatment outcomes.
Another critical method is Survival Analysis, which focuses on time-to-event data, allowing researchers to analyze the duration until significant events occur, such as death or disease progression.
Finally, Meta-Analysis consolidates findings from various studies, revealing trends or overall impacts that strengthen the reliability of conclusions drawn from individual experiments.
By exploring these methodologies, scientists can gain profound insights into effectively utilizing analytical techniques in their trials, ultimately enhancing the quality and impact of statistics in clinical trials.

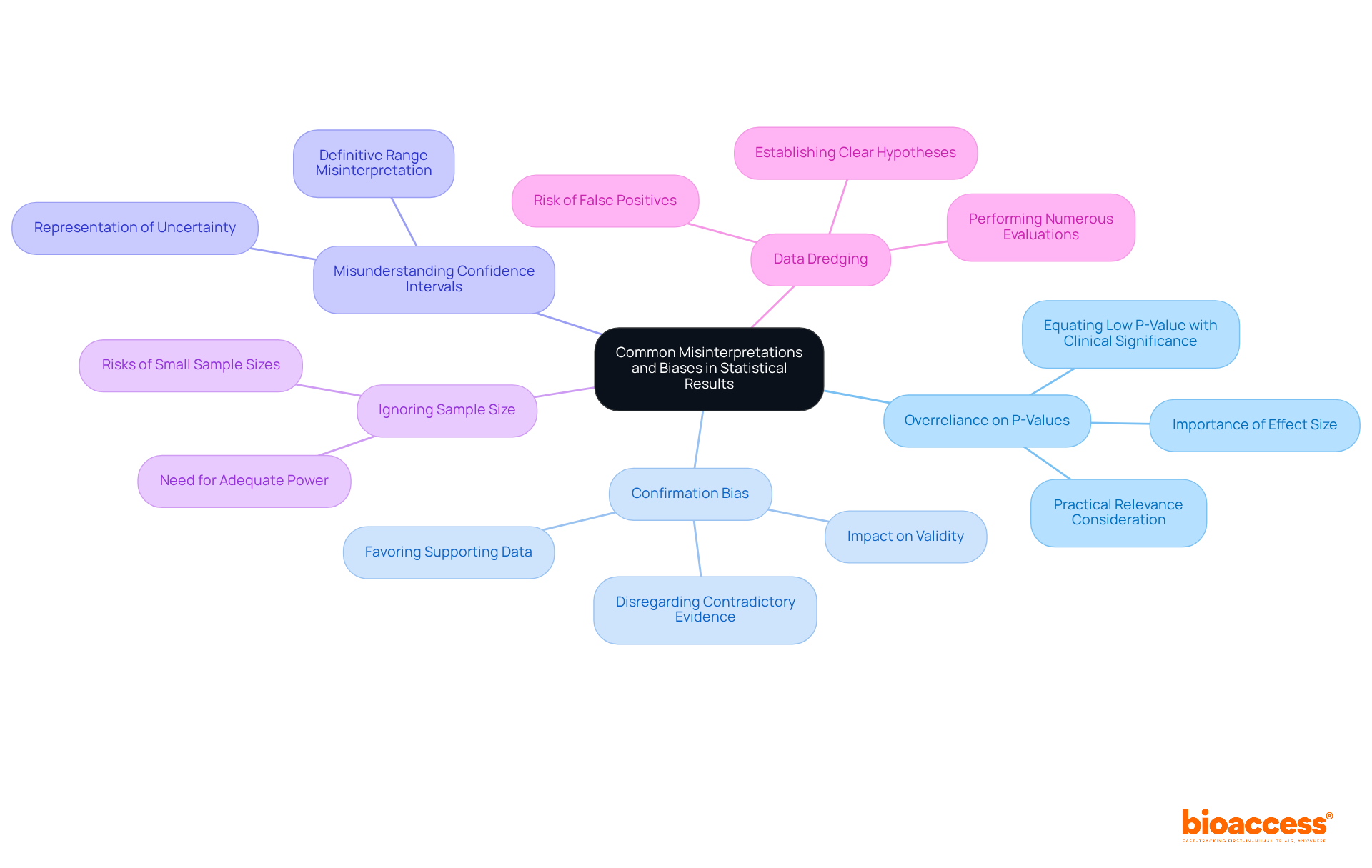
Common misinterpretations and biases in statistical results can significantly impact clinical research outcomes. Understanding these pitfalls is crucial for enhancing the rigor of research methodologies.
By identifying these common misinterpretations and biases, researchers can enhance the rigor and credibility of their statistics in clinical trials analyses. This awareness fosters a more robust framework for interpreting data, ultimately leading to more reliable clinical research outcomes.
Mastering statistics in clinical trials transcends mere academic exercise; it serves as a cornerstone of effective research management, directly influencing the validity and reliability of clinical studies. Grasping essential statistical concepts—such as population, randomization, control groups, and statistical power—is imperative for researchers intent on elevating the quality of their findings and adhering to regulatory standards.
This article has delved into critical insights regarding the significance of statistical analysis. The role of statistics in interpreting complex data sets, validating treatment efficacy, assessing risks, and guiding resource allocation highlights its crucial position in clinical research. Moreover, a variety of statistical methods—including descriptive and inferential statistics, regression analysis, and meta-analysis—arm researchers with the necessary tools to extract meaningful conclusions from their data. Recognizing common misinterpretations and biases, such as overreliance on p-values and confirmation bias, further underscores the necessity for rigorous statistical practices.
In light of these discussions, it is evident that a profound understanding of statistics is vital for propelling clinical research forward. Researchers are urged to:
By prioritizing statistical literacy, the potential to enhance clinical outcomes and expedite the development of effective treatments is significantly magnified, ultimately benefiting the broader healthcare landscape.
What is the definition of population and sample in clinical trials?
The population refers to the complete set of individuals relevant to the study, while a sample is a portion of that population selected for examination. Understanding this distinction is important for generalizing results.
What is the purpose of randomization in clinical trials?
Randomization involves randomly assigning participants to different treatment groups to minimize bias and ensure that the groups are comparable.
What role do control groups play in clinical trials?
Control groups serve as a baseline for comparison to assess the effects of the treatment, helping to understand the treatment's efficacy.
What are p-values and confidence intervals in the context of clinical trials?
P-values indicate the probability that the observed results occurred by chance, while confidence intervals provide a range of values within which the true effect likely lies. Both are essential for interpreting the significance of findings in clinical trials.
What is statistical power in clinical trials?
Statistical power refers to the probability of correctly rejecting the null hypothesis when it is false. A study with high power is more likely to detect a true effect if it exists.
What services do efficient management services for studies provide?
Efficient management services, such as those provided by bioaccess, include feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and thorough reporting.
How do management services contribute to clinical trials?
These services support compliance with INVIMA regulatory functions and enhance the understanding and application of quantitative concepts, aiding researchers in navigating data evaluation in medical trials.