

Mastering the FDA 510(k) database is paramount for efficient clinical research, as it offers critical insights into medical devices that have successfully navigated the premarket notification process. This knowledge is instrumental in demonstrating substantial equivalence for new product applications. Familiarity with the database not only streamlines the application process and boosts the likelihood of approval but also aids in identifying competitors and analyzing market trends. Ultimately, this facilitates better strategic planning within the medical device sector, underscoring the importance of leveraging such resources for informed decision-making.
Mastering the FDA's 510(k) database is essential for any medical equipment manufacturer seeking to navigate the complexities of clinical research and product approval. This database not only provides critical insights into previously cleared devices but also serves as a roadmap for demonstrating substantial equivalence—a key requirement for 510(k) submissions.
With the stakes high and the process fraught with challenges, manufacturers must consider how to effectively leverage this resource to enhance their chances of approval and achieve market success.
The 510 k database FDA serves as a vital resource for manufacturers of medical equipment, offering extensive access to information on products that have successfully navigated the premarket notification process. Mastery of the 510 k database FDA is crucial for demonstrating that a new product is significantly comparable to an existing one, which is a fundamental requirement for 510(k) applications. It encompasses detailed information about cleared equipment, including their intended uses, technological specifications, and performance metrics. Familiarity with this data not only streamlines the application process but also significantly increases the likelihood of obtaining approval.
Moreover, the database is instrumental in identifying potential competitors and analyzing market trends, rendering it an essential tool for strategic planning in clinical research. Recent updates to the FDA's guidance on best practices for predicate product selection underscore the importance of effectively utilizing this database, as it can lead to enhanced outcomes in product development and market entry. Notably, a substantial portion of medical equipment manufacturers rely on the 510 k database FDA to inform their applications, illustrating its extensive utility in the sector.
Importantly, 97% of all products cleared with 510(k) underwent post-marketing safety testing compliance tests, highlighting the critical role of the 510(k) process in ensuring product safety and efficacy. A pertinent example is Abbott’s Freestyle Libre system, which received 510(k) clearance in 2017, showcasing how the 510 k database FDA facilitated its market entry and contributed to its success.
In this context, bioaccess provides comprehensive clinical trial management services that enhance the efficiency of navigating the FDA's 510 k database process. Our capabilities encompass:
All of which are essential for ensuring that clinical trials are conducted smoothly and effectively. By leveraging these services, medical equipment manufacturers can simplify their applications while also fostering local economies through job creation and advancements in healthcare, thereby promoting international cooperation within the medtech industry.
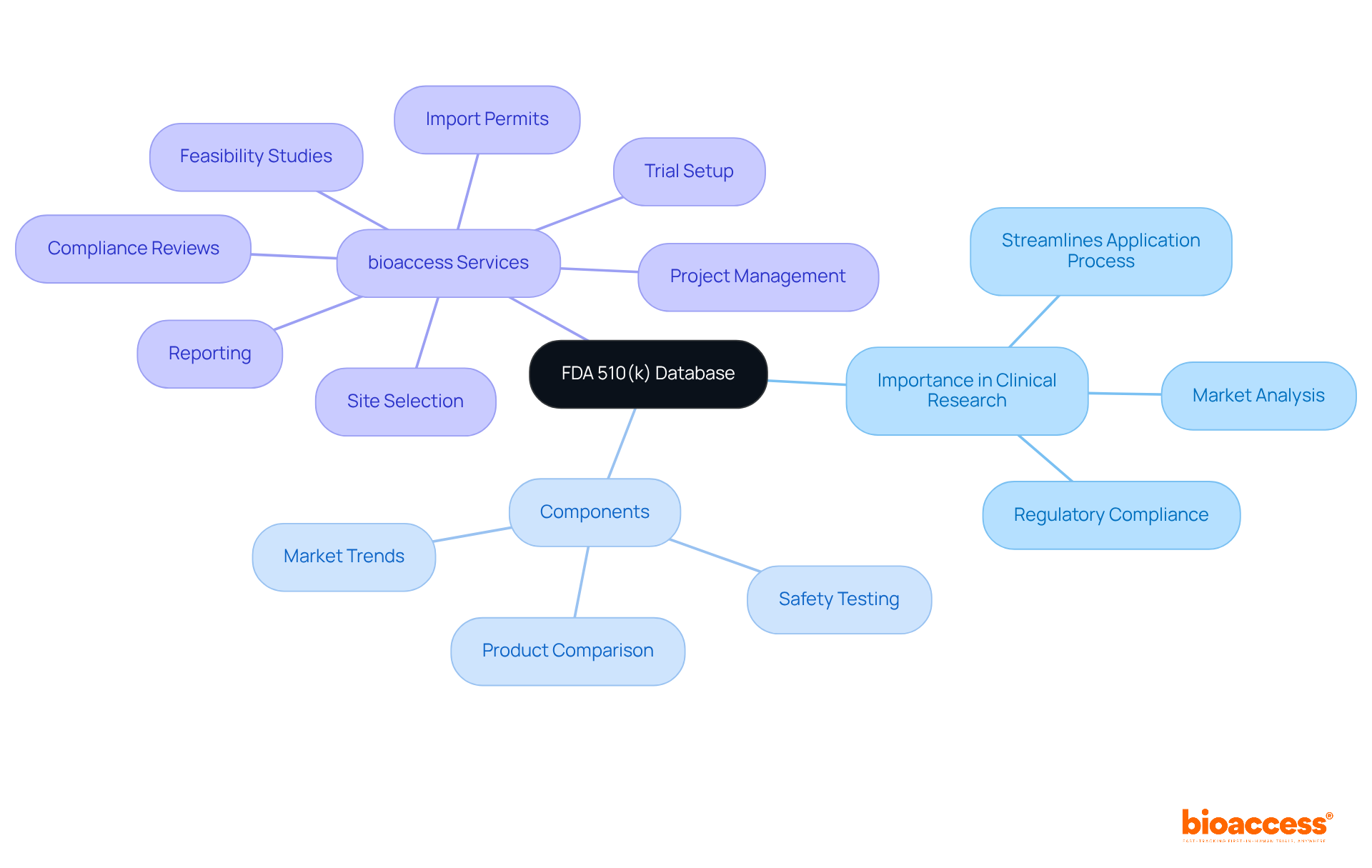
The FDA recognizes three primary types of 510(k) submissions: Traditional, Special, and Abbreviated, each tailored to specific circumstances and requirements:
Traditional 510(k): This is the most prevalent submission type, utilized when a device is deemed substantially equivalent to an existing predicate device. It requires thorough documentation, including detailed descriptions of the equipment, labeling, and performance data. The typical review timeline for a Traditional 510(k) is approximately 90 days, making thorough preparation essential for timely approval.
Special 510(k): Intended for products undergoing minor adjustments to previously authorized items, this filing type simplifies the procedure by focusing on the particular alterations rather than the whole item. The review timeline for a Special 510(k) is significantly shorter, at just 30 days, allowing for quicker market access. However, it is crucial that the modifications can be evaluated using well-established methods, as performance data may not be required in full.
Abbreviated 510(k): This filing is relevant when a product complies with specific criteria, such as being grounded in consensus standards or guidance documents. It simplifies the application process by allowing manufacturers to reference existing data, thus expediting the review.
Comprehending the subtleties of each submission category in the 510 k database fda is essential for guaranteeing adherence and enhancing the efficiency of the approval process, ultimately enabling quicker access to the market for innovative medical products.
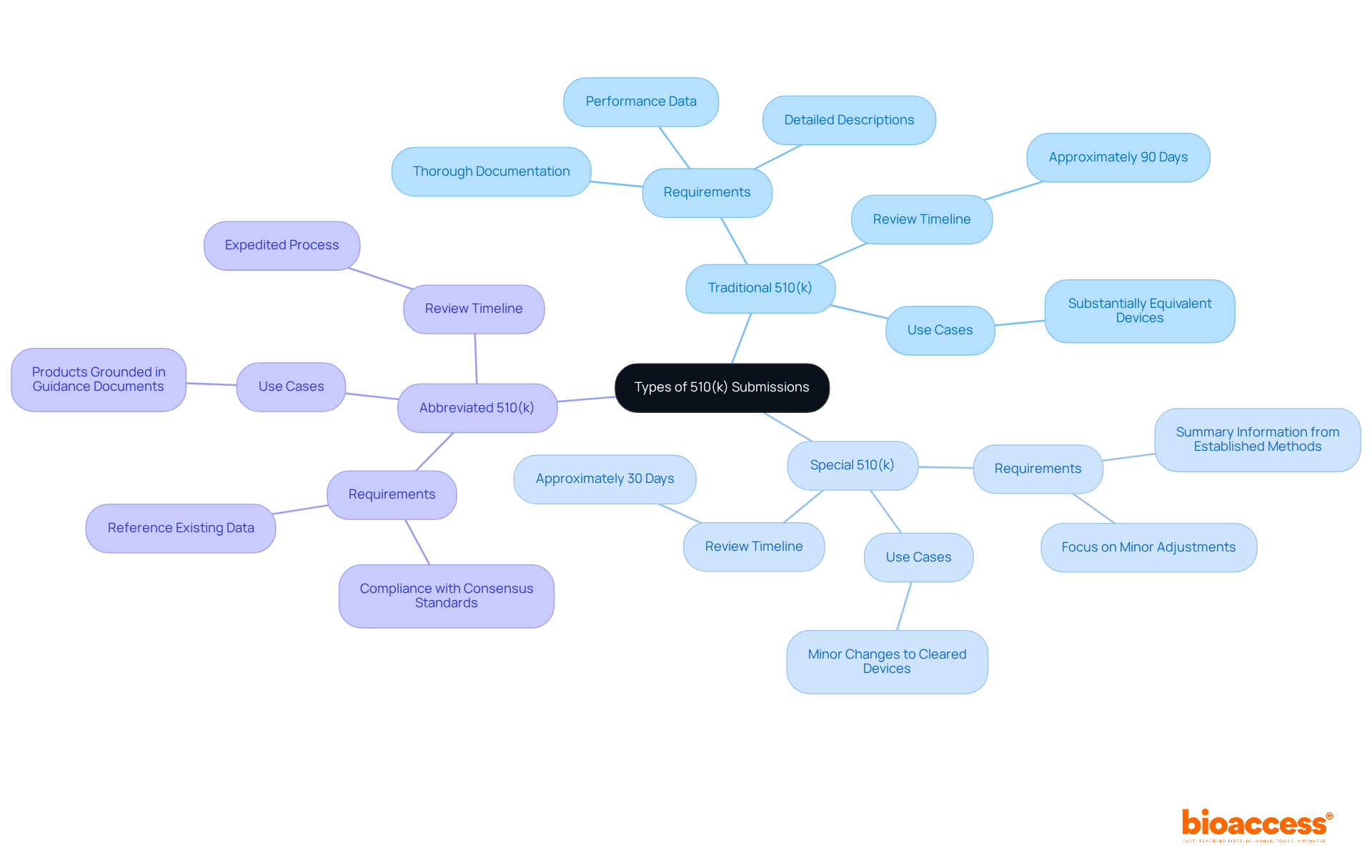
Accessing the 510 k database FDA is crucial for effective clinical research. To navigate it efficiently, follow these steps:
By mastering the navigation of the 510 k database fda, you can collect critical information that informs your approach and enhances your understanding of the competitive landscape, ultimately leading to more efficient clinical research. Furthermore, leveraging comprehensive clinical trial management services like those provided by bioaccess®—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—can significantly streamline your research process. This is particularly relevant in Latin America, where bioaccess® boasts extensive expertise.
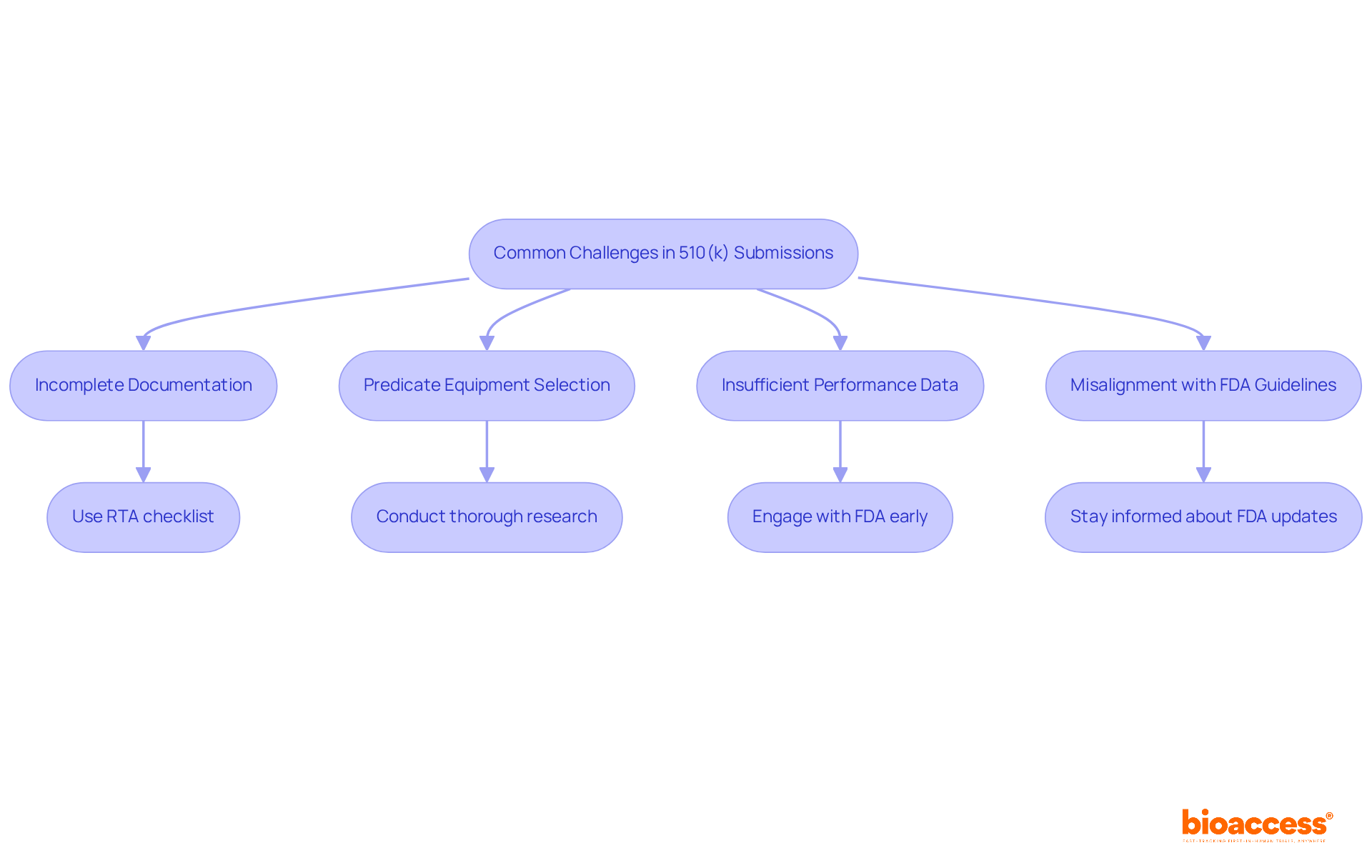
Common challenges in 510(k) submissions include:
Incomplete Documentation: A significant 25% of FDA 510(k) submissions are rejected due to lack of completeness. To mitigate this risk, ensure that all required documents are included in your submission. Utilizing the FDA's Refuse to Accept (RTA) checklist can help verify completeness and prevent unnecessary delays. Engaging a clinical trial management service can provide valuable support in ensuring all documentation is meticulously prepared.
Predicate Equipment Selection: The choice of suitable predicate equipment is crucial for demonstrating substantial equivalence. Conduct thorough research to identify a predicate that closely aligns with your equipment's intended use and technological characteristics. Successful predicate selection can significantly enhance the likelihood of approval. Bioaccess offers expertise in feasibility studies and site selection, which can aid in identifying suitable predicates based on clinical data.
Insufficient Performance Data: Robust clinical information is essential for instruments requiring such evidence. Engage with the FDA early through pre-submission meetings to clarify expectations and ensure that your performance data meets regulatory standards. This proactive method can assist in tackling possible deficiencies prior to handing in. With Katherine Ruiz's expertise in regulatory affairs for medical equipment and in vitro diagnostics in Colombia, bioaccess can aid in collecting and showcasing the essential performance data efficiently.
Misalignment with FDA Guidelines: Staying informed about the latest FDA guidance documents is vital. Regularly review updates to ensure that your entry aligns with current requirements, as misalignment can lead to rejection.
By proactively addressing these challenges, you can streamline your submission process and significantly improve your chances of obtaining timely clearance for your device via the 510 k database fda. Enhanced communication with the FDA and educational initiatives can further support manufacturers in navigating these complexities effectively. Leveraging the comprehensive clinical trial management services from bioaccess can also provide a strategic advantage in overcoming these hurdles.
Mastering the FDA 510(k) database is not just beneficial; it is essential for efficient clinical research and successful medical device development. This guide underscores the critical importance of understanding the database, which serves as an invaluable resource for manufacturers to demonstrate substantial equivalence and streamline the application process. By leveraging the wealth of information available, researchers can significantly enhance their chances of obtaining approvals and effectively navigate the competitive landscape of medical technology.
Key insights from this article emphasize the necessity of selecting the appropriate type of 510(k) submission—Traditional, Special, or Abbreviated—based on the specific circumstances surrounding the device. Furthermore, the guide provides practical steps for accessing and navigating the 510(k) database while addressing common challenges that may arise during the submission process. By grasping these elements, manufacturers can substantially improve their submission efficiency and ensure compliance with FDA guidelines.
Ultimately, the effective use of the FDA 510(k) database not only accelerates the path to market for innovative medical devices but also reinforces a commitment to safety and efficacy in healthcare. Engaging with clinical trial management services, such as those offered by bioaccess, can further streamline this process, fostering advancements in medical technology and enhancing patient outcomes. Embracing these strategies will empower manufacturers to navigate the complexities of the regulatory landscape and contribute to the ongoing evolution of healthcare solutions.
What is the FDA 510(k) database?
The FDA 510(k) database is a vital resource for manufacturers of medical equipment, providing access to information on products that have successfully completed the premarket notification process.
Why is mastery of the FDA 510(k) database important?
Mastery of the 510(k) database is crucial for demonstrating that a new product is significantly comparable to an existing one, which is a fundamental requirement for 510(k) applications.
What type of information can be found in the FDA 510(k) database?
The database includes detailed information about cleared medical equipment, such as their intended uses, technological specifications, and performance metrics.
How does familiarity with the 510(k) database benefit manufacturers?
Familiarity with the database streamlines the application process and significantly increases the likelihood of obtaining approval for new products.
In what ways can the 510(k) database assist in market analysis?
The database helps identify potential competitors and analyze market trends, making it an essential tool for strategic planning in clinical research.
What recent updates have been made regarding the FDA's guidance on the 510(k) process?
Recent updates emphasize the importance of effectively utilizing the 510(k) database for predicate product selection, which can enhance outcomes in product development and market entry.
How prevalent is the use of the 510(k) database among medical equipment manufacturers?
A substantial portion of medical equipment manufacturers rely on the 510(k) database to inform their applications, showcasing its extensive utility in the sector.
What percentage of products cleared with 510(k) underwent post-marketing safety testing?
97% of all products cleared with 510(k) underwent post-marketing safety testing compliance tests, highlighting the critical role of the 510(k) process in ensuring product safety and efficacy.
Can you provide an example of a product that successfully used the 510(k) database?
Abbott’s Freestyle Libre system, which received 510(k) clearance in 2017, is an example of how the 510(k) database facilitated market entry and contributed to product success.
What services does Bioaccess provide to enhance the efficiency of navigating the FDA's 510(k) database process?
Bioaccess offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
How do these services benefit medical equipment manufacturers?
By leveraging these services, manufacturers can simplify their applications, foster local economies through job creation, and promote advancements in healthcare and international cooperation within the medtech industry.