


The primary focus of the article "Master the Calculation of Sample Size in Clinical Trials" is to underscore the critical significance and methodology of accurately calculating sample size in clinical trials, which is essential for ensuring reliable and valid research outcomes. It elaborates on key components such as:
It illustrates how precise calculations can avert underpowered studies, ethical dilemmas, and resource wastage. Ultimately, this enhances the credibility of clinical research, making it imperative for professionals in the field.
The calculation of sample size in clinical trials stands as a pivotal element that can decisively influence the validity of research outcomes. It not only determines the number of participants required to attain statistically significant results but also holds significant ethical implications, ensuring that participants are not subjected to unnecessary risks.
As researchers navigate the complexities inherent in clinical trials, a crucial question emerges: how can they accurately ascertain the optimal sample size that balances statistical power with ethical responsibility while steering clear of common pitfalls?
This article explores the intricacies of sample size calculation, providing a step-by-step guide to mastering this essential skill in clinical research.
The calculation of sample size in clinical trials is a cornerstone of clinical trial design, directly influencing the reliability and validity of research outcomes. It determines the number of participants required to achieve statistically significant results while minimizing the risks of Type I and Type II errors. For instance, to identify a mean weight difference of 700 grams between two groups of newborns, a group of 82 patients per category is essential at a 95% confidence level and 80% power. However, should the standard deviation be assumed to be 30, the number of participants needed rises to 190 individuals per group, illustrating how variability can significantly impact research outcomes.
A well-planned group of participants not only guarantees adequate power to identify genuine effects but also enhances the reliability of the results. Recent research indicates that underpowered trials frequently yield inconclusive outcomes, whereas excessively large groups can squander resources and introduce biases. For instance, the DREAMS research, which aimed to decrease postoperative vomiting, initially estimated a participant count of 950 individuals after factoring in a 10% dropout rate. This highlights the significance of predicting participant loss in research design.
Ethical considerations further underscore the importance of precise participant number estimations. Researchers must avoid exposing unnecessary participants to potential risks, making it imperative to balance statistical significance with ethical responsibility. As indicated in various studies, overlooking appropriate estimation of participant numbers can lead to the rejection of effective treatments or the endorsement of ineffective ones, thus emphasizing the ethical considerations surrounding this essential element of clinical research. Therefore, mastering the calculation of the number of participants is crucial for executing responsible and impactful clinical trials.

When calculating sample size, several key components must be considered:
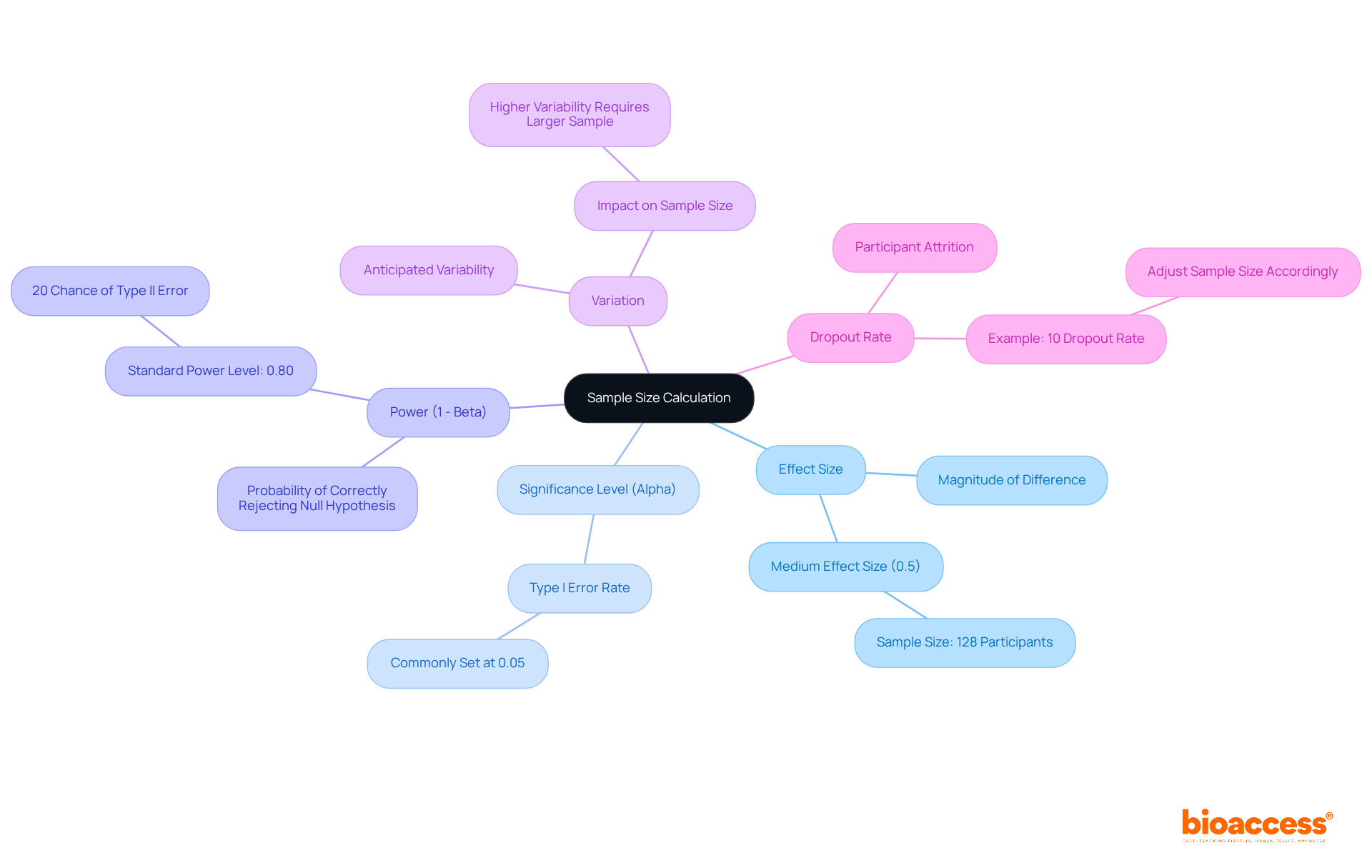
Effect Size: This represents the magnitude of the difference anticipated between groups. A larger effect magnitude typically enables a smaller group to identify the effect. For instance, to attain a power of 80% for a medium effect magnitude (0.5), a total sample of 128 participants is necessary, with 64 in each group. This relationship emphasizes the significance of precisely assessing effect size in the calculation of sample size in clinical trials to prevent underpowered research.
Significance Level (Alpha): This is the probability of committing a Type I error, commonly set at 0.05, which indicates a 5% chance of falsely rejecting the null hypothesis. This threshold is essential for establishing statistical significance in research results and should be thoughtfully evaluated in relation to the aims of the investigation.
Power (1 - Beta): Power is the probability of correctly rejecting the null hypothesis, reflecting the study's ability to detect a true effect. A power level of 0.80 is standard, implying a 20% chance of a Type II error (failing to detect an effect when one exists). As the number of observations grows, statistical power enhances, decreasing the chances of Type II errors.
Variation: The anticipated variability within the data greatly affects the requirements for the number of observations. Increased variability requires a larger group to guarantee dependable and accurate outcomes. For instance, if the expected dropout rate is approximately 10%, modifications need to be implemented to the initial group to preserve sufficient power.
Dropout Rate: Predicting participant attrition is crucial in the calculation of participants. To avoid underpowered investigations, researchers should take into account the calculation of sample size in clinical trials, particularly considering anticipated attrition rates. For example, if research expects a dropout rate of 10%, the number of subjects should be increased accordingly to ensure that the final count of participants remains adequate for analysis. As noted by Alaa Althubaiti, researchers must face the reality that not all invited participants are willing to be enrolled, which entails the possibility of a low response rate.
By carefully considering these components, including the calculation of sample size in clinical trials, researchers can enhance the robustness of their clinical trials, ensuring that their findings are both statistically significant and clinically relevant. Furthermore, it is advised that clinical trials target a minimum number of at least 100 participants per group, and that effect estimates and 95% confidence intervals be disclosed alongside p-values for significant interpretation.
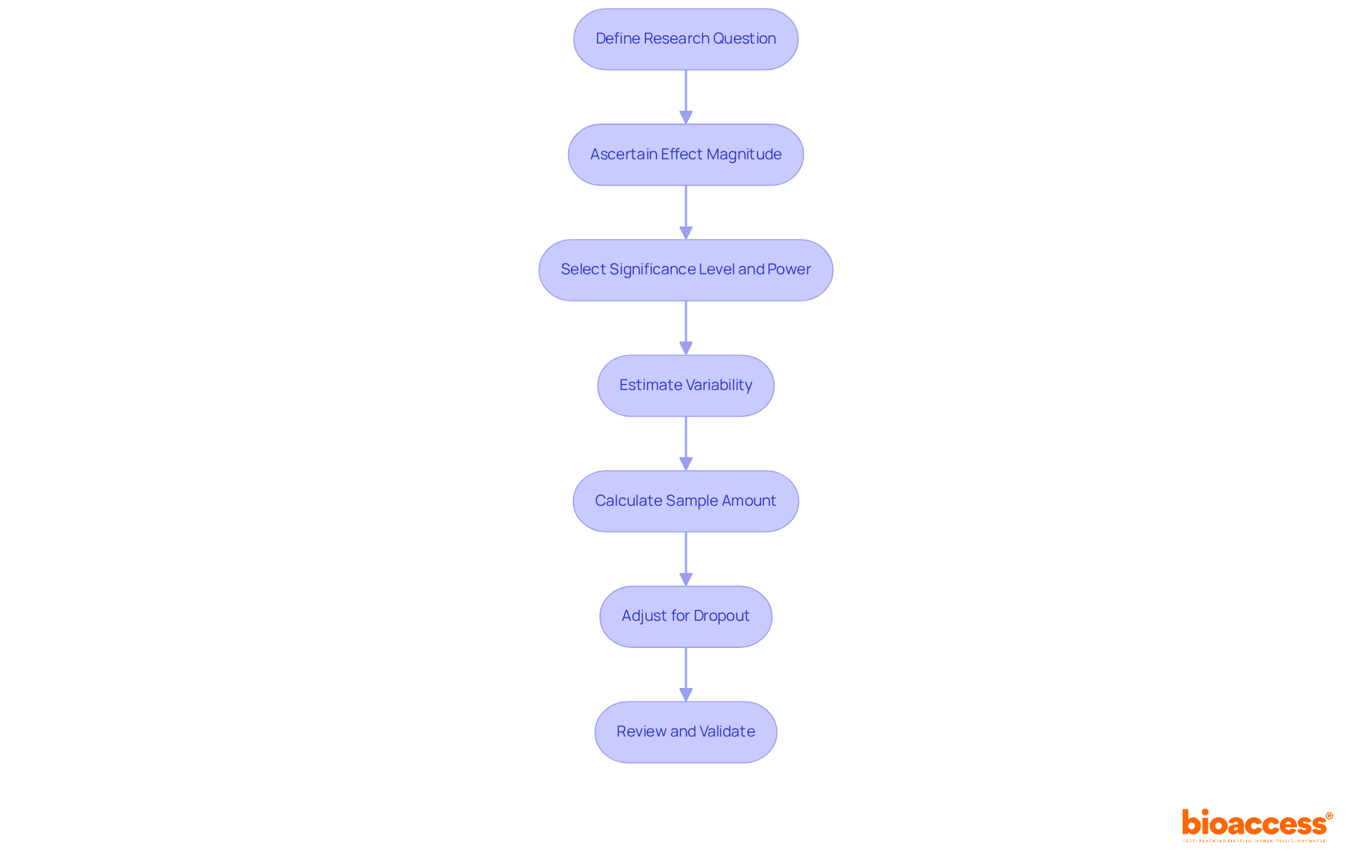
To calculate sample size effectively, follow these structured steps:
n = (Zα/2 + Zβ)² * (σ1² + σ2²) / (μ1 - μ2)²
where Zα/2 is the Z-score for the desired alpha level, Zβ is the Z-score for the desired power, σ1 and σ2 are the standard deviations, and μ1 and μ2 are the means of the two groups. For large populations, Cochran's formula can be particularly useful. 6. Adjust for Dropout: Anticipate potential dropout rates and adjust the calculated sample size accordingly using the formula:
Adjusted Sample Size = n / (1 - dropout rate)
It is recommended to allow for a non-response rate of 20% to 30%. 7. Review and Validate: Collaborate with a biostatistician to confirm your computations and assumptions. This collaboration ensures robustness in your research design.
By adhering to these procedures, researchers can guarantee that their calculation of sample size in clinical trials is both precise and consistent with their research goals, ultimately enhancing the credibility of their clinical trials.
Sample size calculation presents several significant challenges:
Estimating Effect Magnitude: Accurately assessing effect magnitude is vital; miscalculations can result in research that is either underpowered or overpowered. For instance, when the effect magnitude is 0.2, even a group of 30 is inadequate to achieve a significant power value. Conducting pilot experiments is essential for enhancing these estimates, allowing researchers to collect preliminary information that informs more accurate assessments.
Variability in Data: The considerable variability in clinical trial data complicates participant count calculations. Reducing the standard deviation (sd) from 25 μm to 20 μm decreases the required number of subjects to 128 (64 subjects per treatment group). Utilizing historical data or performing initial analyses can yield improved estimates of variability, which is crucial for determining the suitable sample size necessary to obtain reliable results.
Dropout Rates: Anticipating dropout rates poses a significant challenge. It is crucial to base estimates on prior research or comparable trials in the calculation of sample size in clinical trials to make informed adjustments. Furthermore, sponsors should assess and summarize patterns of pandemic-related missing data in affected trials to better understand dropout implications. For example, if historical information indicates a 20% dropout rate, this should be factored into the determination of the group size needed to maintain statistical strength.
Complex Research Designs: Multi-arm trials or investigations with intricate endpoints may require advanced statistical techniques for calculating sample sizes. In such scenarios, consulting with a biostatistician is advisable to ensure that all relevant factors are considered and that the study is adequately powered. Employing sophisticated statistical methods is essential for the calculation of sample size in clinical trials in these complex situations.
Regulatory Considerations: Various regulatory organizations may impose specific criteria for determining the number of subjects. Familiarizing oneself with these guidelines is imperative to ensure compliance and to avoid potential delays in the approval process.
Software Limitations: While numerous software tools exist for determining sample size, such as nQuery, which encompasses over 1000 sample size procedures, they may not account for all variables pertinent to your study. Understanding the underlying statistical principles is essential for accurate calculations, as reliance solely on software can lead to oversights in critical assumptions.

The calculation of sample size in clinical trials stands as a fundamental pillar for ensuring the integrity and validity of research outcomes. By accurately determining the number of participants required, researchers can effectively minimize errors and achieve statistically significant results. This process not only enhances the reliability of findings but also aligns with ethical considerations, safeguarding participants from unnecessary risks while maximizing the potential for impactful discoveries.
Key components such as effect size, significance level, power, variability, and dropout rates are critical factors influencing sample size calculations. Each element plays a vital role in shaping the research design, ensuring that studies are adequately powered to detect true effects while avoiding the pitfalls of underpowered or overpowered trials. The outlined step-by-step approach provides a clear roadmap for researchers, emphasizing the importance of thorough planning and validation in the calculation process.
Ultimately, mastering sample size calculation transcends a mere statistical exercise; it is an essential practice that can significantly impact clinical research outcomes. Researchers are encouraged to prioritize this aspect of trial design, utilizing available resources and expert consultations to navigate common challenges. By doing so, they can contribute to the advancement of medical knowledge and the development of effective treatments, reinforcing the notion that meticulous planning in sample size determination is crucial for the success of clinical trials.
Why is sample size calculation important in clinical trials?
Sample size calculation is crucial because it directly influences the reliability and validity of research outcomes, determining the number of participants needed to achieve statistically significant results while minimizing Type I and Type II errors.
How does variability affect the required sample size?
Variability, such as standard deviation, can significantly impact the required sample size. For example, to detect a mean weight difference of 700 grams between two groups, 82 participants per group are needed at a 95% confidence level and 80% power. However, if the standard deviation is assumed to be 30, the number of participants needed increases to 190 per group.
What are the consequences of underpowered trials?
Underpowered trials often yield inconclusive outcomes, making it difficult to identify genuine effects. This can lead to ineffective treatments being endorsed or effective treatments being rejected.
How do excessively large participant groups affect research?
Excessively large groups can waste resources and introduce biases into the study, which can compromise the integrity of the research findings.
What ethical considerations are associated with sample size calculation?
Ethical considerations involve avoiding unnecessary exposure of participants to potential risks. Researchers must balance the need for statistical significance with their ethical responsibility to protect participants.
What can happen if participant number estimations are overlooked?
Overlooking appropriate estimations can lead to the rejection of effective treatments or the endorsement of ineffective ones, underscoring the ethical implications of accurate sample size calculations in clinical research.
How does participant dropout rate influence sample size estimation?
Participant dropout rates must be factored into sample size estimations to ensure that the planned number of participants remains adequate throughout the study. For instance, the DREAMS research initially estimated a participant count of 950, accounting for a 10% dropout rate.