


This article serves as a comprehensive step-by-step guide to mastering the Certification of Suitability (CEP), highlighting its essential role in regulatory submissions for pharmaceutical products in Europe. Understanding the CEP is not just beneficial; it is critical for ensuring compliance and enhancing the credibility of submissions, which ultimately facilitates market access.
The guide details the application process, emphasizing the importance of proper documentation and the nuances of different types of CEPs. By providing these insights, it underscores how a thorough grasp of the CEP can streamline compliance efforts. This knowledge is invaluable for professionals navigating the complexities of regulatory requirements in the pharmaceutical landscape.
In summary, mastering the CEP is a strategic move for anyone involved in pharmaceutical submissions. It not only aids in compliance but also positions submissions favorably in the eyes of regulatory bodies, paving the way for successful market entry.
Navigating the complex landscape of pharmaceutical compliance can be daunting, especially regarding the certification of suitability (CEP). This crucial document not only validates the quality of active pharmaceutical ingredients but also streamlines the regulatory submission process, significantly impacting market access. As the industry faces evolving regulations and increased scrutiny, manufacturers must ask: how can they master the CEP application and renewal process? This guide explores the intricacies of CEP, offering a step-by-step approach to mastering certification, overcoming common challenges, and ultimately securing a competitive edge in the pharmaceutical market.
The certification of suitability (CEP) is an essential document provided by the European Directorate for the Quality of Medicines & HealthCare (EDQM). It confirms the suitability of a substance for use in the manufacture of medicinal products, particularly active pharmaceutical ingredients (APIs). This certification of suitability is essential for demonstrating compliance with European pharmacopoeia standards, ensuring that the quality of the substance meets the required safety and efficacy benchmarks.
To master the CEP process, it’s vital to understand its purpose. The CEP serves as a key component in compliance submissions, providing a certification of suitability that attests to the quality of your product. Familiarizing yourself with the CEP's function is the first step toward successful certification and market entry for your products. By grasping the significance of the CEP, you position yourself to navigate the complexities of clinical research effectively.
The certification of suitability (CEP) plays a pivotal role in compliance submissions, especially in Europe, as it certifies that the Active Pharmaceutical Ingredient (API) adheres to the quality standards set by the European Pharmacopoeia. Including a certification of suitability in your submission can significantly reduce documentation requirements, streamlining the compliance process. For example, an Indian API manufacturer received a CEP within 18 months after addressing additional data requests related to impurity qualifications, demonstrating how a CEP can expedite the review timeline. This not only accelerates approval but also bolsters the credibility of your submission, making the certification of suitability an indispensable component of your compliance strategy.
As the pharmaceutical landscape evolves in 2025, with new requirements for risk evaluations due to nitrosamine controls, the importance of the certification of suitability (CEP) in ensuring compliance and facilitating smoother oversight pathways cannot be overstated. Industry leaders assert that mastering CEP filings enhances submission credibility and aligns with the latest regulatory expectations, ultimately supporting successful market access and the certification of suitability. Furthermore, it’s essential to remember that the certification of suitability for CEPs must be renewed every five years to maintain their validity, ensuring ongoing compliance with evolving standards.
Understanding the various types of certification of suitability is essential in the realm of clinical research. These certificates include the certification of suitability for active substances used in medicinal products and for excipients, each serving a distinct purpose and governed by specific regulations. For example, the certification of suitability for an active substance typically demands more stringent documentation than one for an excipient.
Familiarizing yourself with these distinctions is crucial. It enables you to select the correct type of CEP that aligns with your product's requirements. This understanding not only guides you in preparing the necessary documentation for certification of suitability but also ensures compliance with the relevant regulations. By grasping these nuances, you position yourself to navigate the complexities of the Medtech landscape effectively.
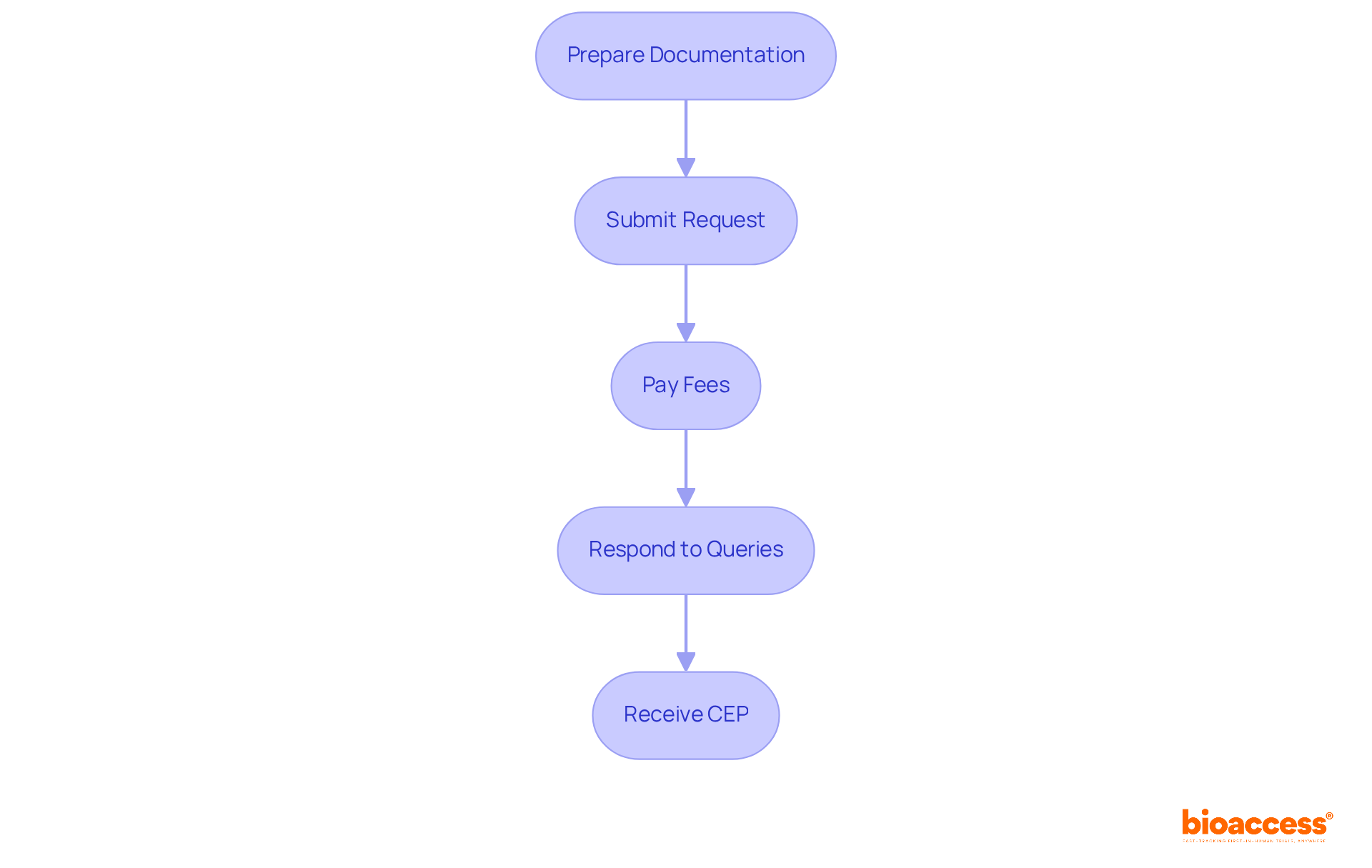
To apply for a CEP, follow these essential steps:
Adhering to these steps diligently will significantly improve your chances of a successful submission.
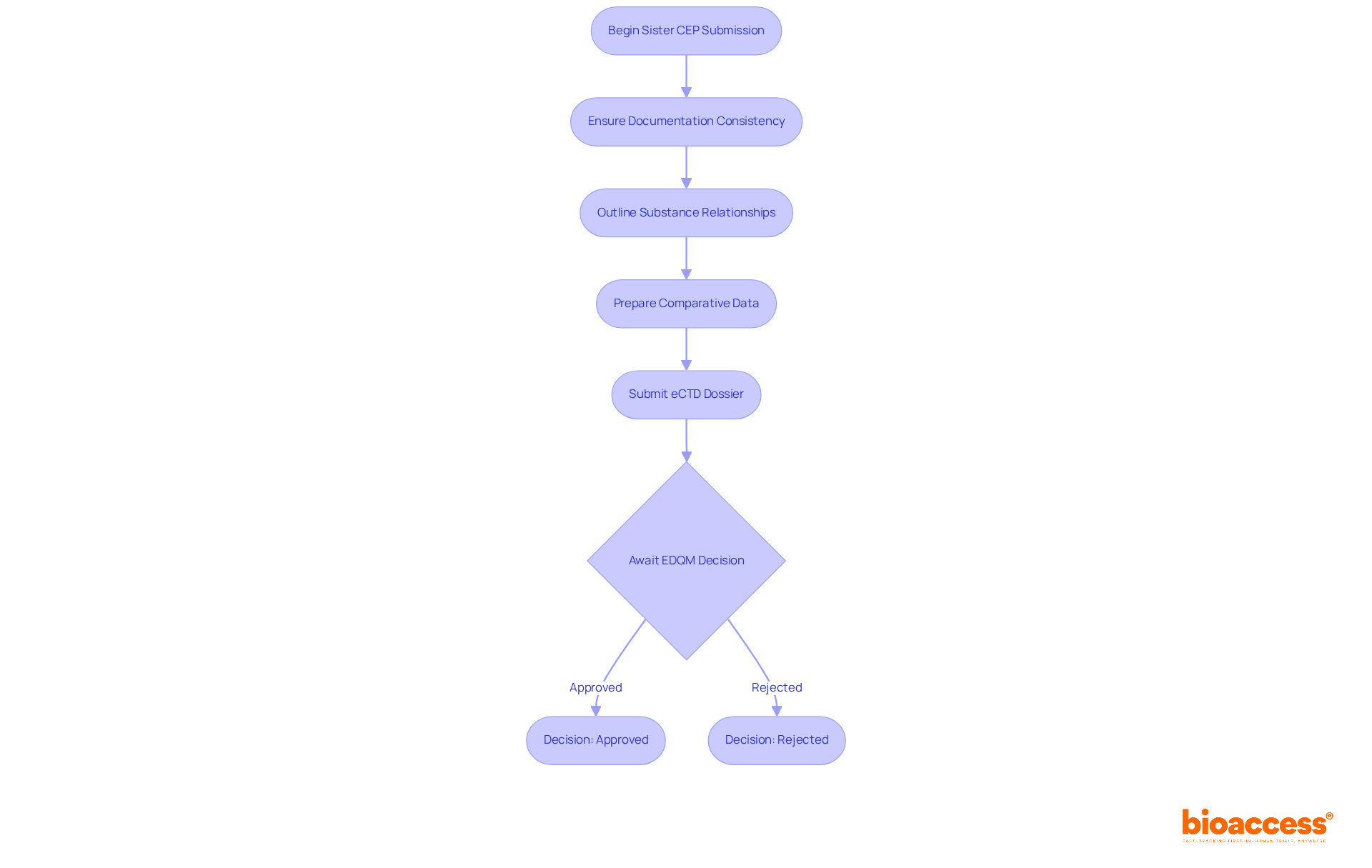
Sister CEP submissions are pivotal in the realm of clinical research, as they refer to applications for multiple certification of suitability that are interconnected, such as those for different grades of the same active substance. When submitting sister CEPs, it’s essential to ensure that all related documentation is consistent and that each submission clearly outlines the relationship between the substances involved. Moreover, comparative data may be necessary to demonstrate that all substances meet the required quality standards. Understanding these requirements is vital for ensuring the certification of suitability, avoiding delays in the approval process, and maintaining compliance with regulatory standards.
The sister CEP submission method offers a fast-track procedure, facilitating harmonized assessments that can significantly reduce approval times. This streamlined approach not only simplifies the submission journey but also enhances the efficiency of the process. Successful examples from the pharmaceutical sector illustrate how producers have adeptly managed these submissions, leveraging existing CEPs to strengthen their proposals. However, common challenges persist, such as maintaining consistent documentation and addressing discrepancies that may arise during the review process.
Regulatory specialists emphasize the importance of meticulous documentation management for the certification of suitability when handling multiple CEP submissions. As highlighted by the EDQM, "CEPs should be renewed once after five years of the issue date of the original CEP." Consistency not only paves the way for smoother approvals but also builds trust with oversight organizations, ultimately facilitating quicker market access for innovative pharmaceutical products. Additionally, preparing a comprehensive dossier in the electronic Common Technical Document (eCTD) format is a critical requirement for sister CEP submissions. Ultimately, the decision to approve or deny a sister CEP submission rests with the EDQM, underscoring the necessity of adhering to all legal standards.
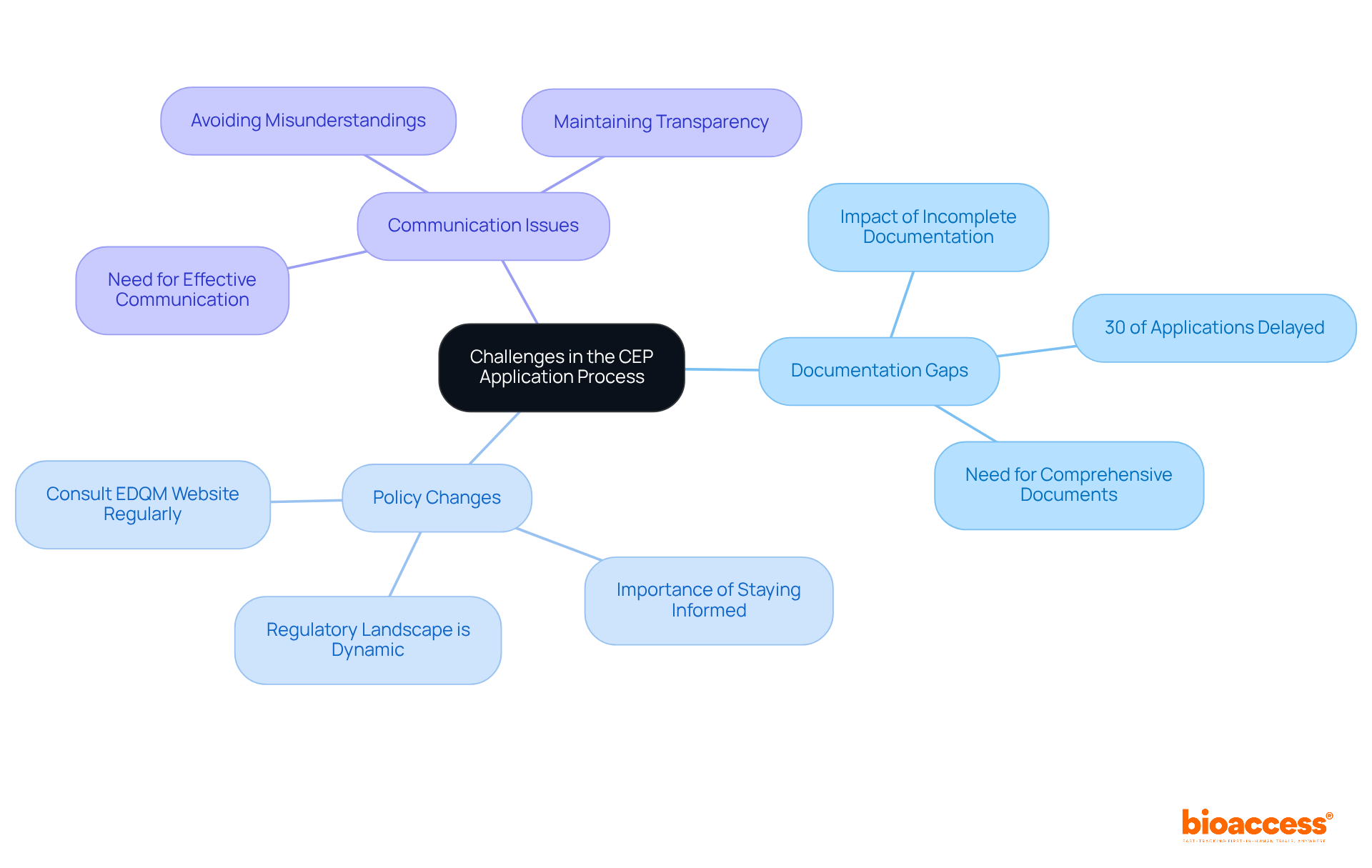
Navigating the CEP application process can be challenging, presenting several hurdles that demand careful attention:
By proactively identifying these challenges, you can implement strategies to minimize their impact, leading to a more efficient and successful CEP application.
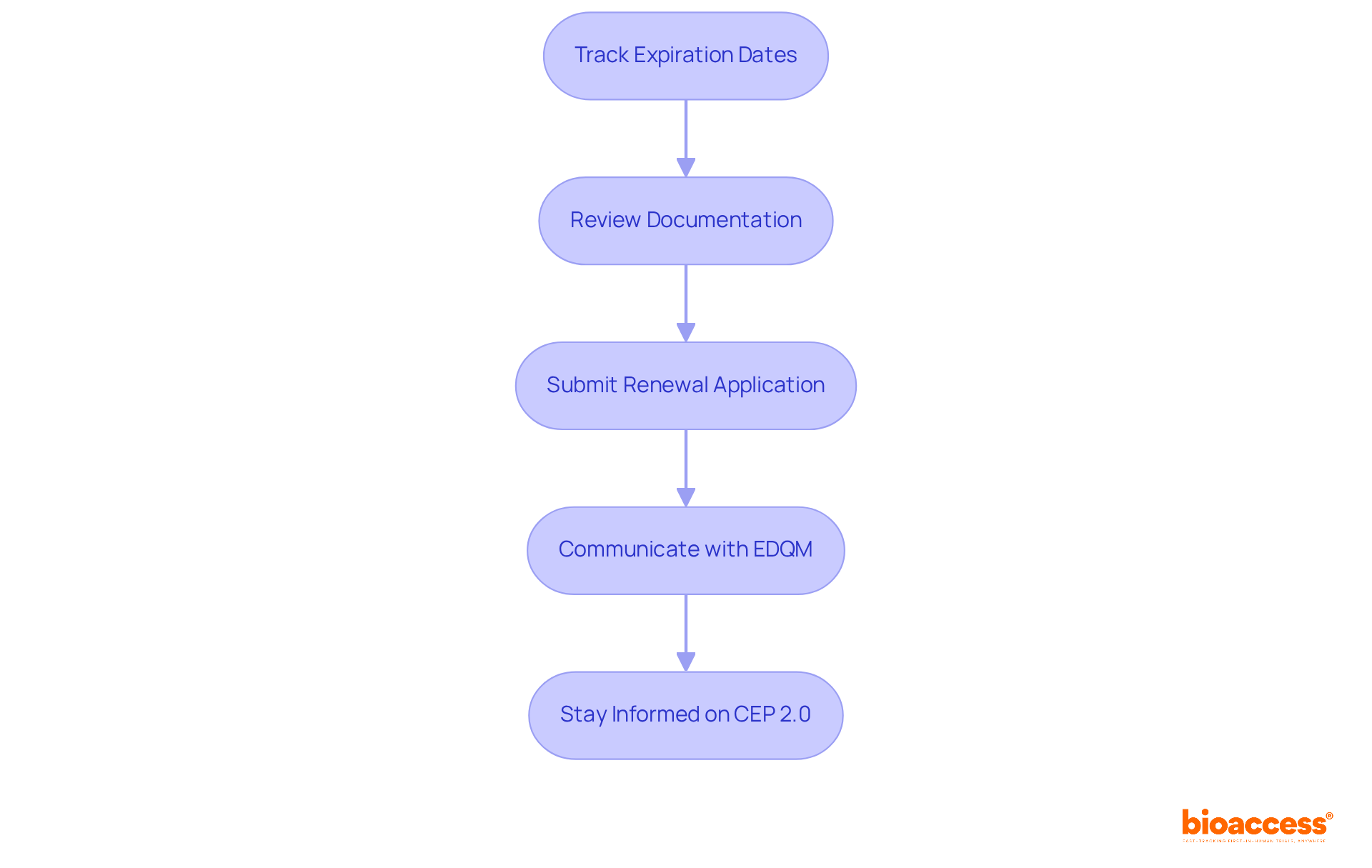
To manage CEP renewals effectively, consider the following steps:
Track expiration dates by maintaining a comprehensive calendar that lists expiration dates for all certification of suitability (CEPs). Recent surveys indicate that only 60% of companies actively track these dates, highlighting a significant area for improvement. According to the European Directorate for the Quality of Medicines & HealthCare (EDQM), good reliance practices are essential for effective regulation of medical products.
Review Documentation: Before renewal, carefully examine all relevant documentation to confirm it is up to date and precisely represents any modifications in manufacturing methods or regulatory requirements. This step is essential for compliance and obtaining the certification of suitability can prevent delays in the renewal procedure.
Submit Renewal Application: Follow the same application procedure as the initial submission, ensuring that all required documents are included. This consistency aids in simplifying the renewal procedure and lowers the chance of oversight.
Communicate with EDQM: Establish and maintain open lines of communication with the European Directorate for the Quality of Medicines & HealthCare (EDQM). Addressing any questions or concerns promptly can facilitate a smoother renewal process.
Stay Informed on CEP 2.0: Be aware of the upcoming changes to the CEP format, which will be implemented from September 2023. The new CEP 2.0 will feature a revised numbering system and a letter of access replacing the declaration of access box, enhancing the sharing of CEPs with customers.
By proactively managing renewals and implementing these best practices, you can ensure that your products remain compliant and available in the market without interruption. Successful strategies for tracking CEP expiration dates include utilizing digital tools and reminders, which have proven effective for many organizations in maintaining compliance.
Mastering the certification of suitability (CEP) is essential for any pharmaceutical entity looking to navigate the complexities of regulatory compliance in Europe. This vital document not only confirms the quality of active pharmaceutical ingredients (APIs) but also streamlines the submission process, enhancing the credibility and efficiency of regulatory applications. Recognizing the importance of the CEP can significantly influence the success of product approvals and market access.
This article explores various facets of the CEP process, including its significance in regulatory submissions, the different types of certifications, and the step-by-step application procedure. It also addresses the challenges encountered during the application process and offers strategies for effectively managing CEP renewals. By covering these key points, it becomes clear that a comprehensive understanding of the CEP landscape is crucial for ensuring ongoing compliance and operational success in the pharmaceutical industry.
Ultimately, the certification of suitability is not merely a regulatory requirement; it serves as a strategic asset that can facilitate smoother market entry and enhance a company's reputation. As the pharmaceutical landscape continues to evolve, staying informed about the latest developments and best practices related to the CEP is essential. Engaging proactively with the certification process—from initial application to timely renewals—empowers organizations to maintain compliance and drive innovation in an ever-competitive market.
What is the certification of suitability (CEP)?
The certification of suitability (CEP) is a document provided by the European Directorate for the Quality of Medicines & HealthCare (EDQM) that confirms the suitability of a substance for use in the manufacture of medicinal products, particularly active pharmaceutical ingredients (APIs). It demonstrates compliance with European pharmacopoeia standards.
Why is the CEP important for regulatory submissions?
The CEP is crucial for regulatory submissions as it certifies that the API meets the quality standards set by the European Pharmacopoeia. Including a CEP can reduce documentation requirements and streamline the compliance process, enhancing the credibility of the submission.
How does the CEP impact the approval timeline for pharmaceutical products?
The CEP can expedite the review timeline for compliance submissions. For instance, an Indian API manufacturer received a CEP within 18 months after addressing additional data requests, showcasing how a CEP can accelerate approval processes.
What are the future implications of CEP in the pharmaceutical industry?
As the pharmaceutical landscape evolves, particularly with new requirements for risk evaluations due to nitrosamine controls, the importance of the CEP in ensuring compliance and facilitating oversight pathways will increase. Mastering CEP filings is essential for maintaining submission credibility and aligning with regulatory expectations.
How often must the certification of suitability be renewed?
The certification of suitability (CEP) must be renewed every five years to maintain its validity and ensure ongoing compliance with evolving standards.