


The article delineates a systematic approach to mastering the clinical development phase, specifically detailing the four stages—Stage I through Stage IV—each vital for evaluating the safety, efficacy, and long-term effects of new drugs. It underscores the significance of adeptly navigating these stages, alongside effective recruitment strategies, regulatory compliance, and adherence to ethical standards. Supported by statistics on completion rates and timelines, these elements collectively contribute to enhancing the success and efficiency of clinical trials.
Understanding the clinical development phase is crucial for anyone involved in drug development. This phase encompasses a systematic approach divided into four key stages, each with its own objectives and challenges. This guide offers a comprehensive step-by-step exploration of these phases, providing insights into the intricacies of ensuring safety, efficacy, and regulatory compliance throughout the process. Navigating this complex landscape raises critical questions:
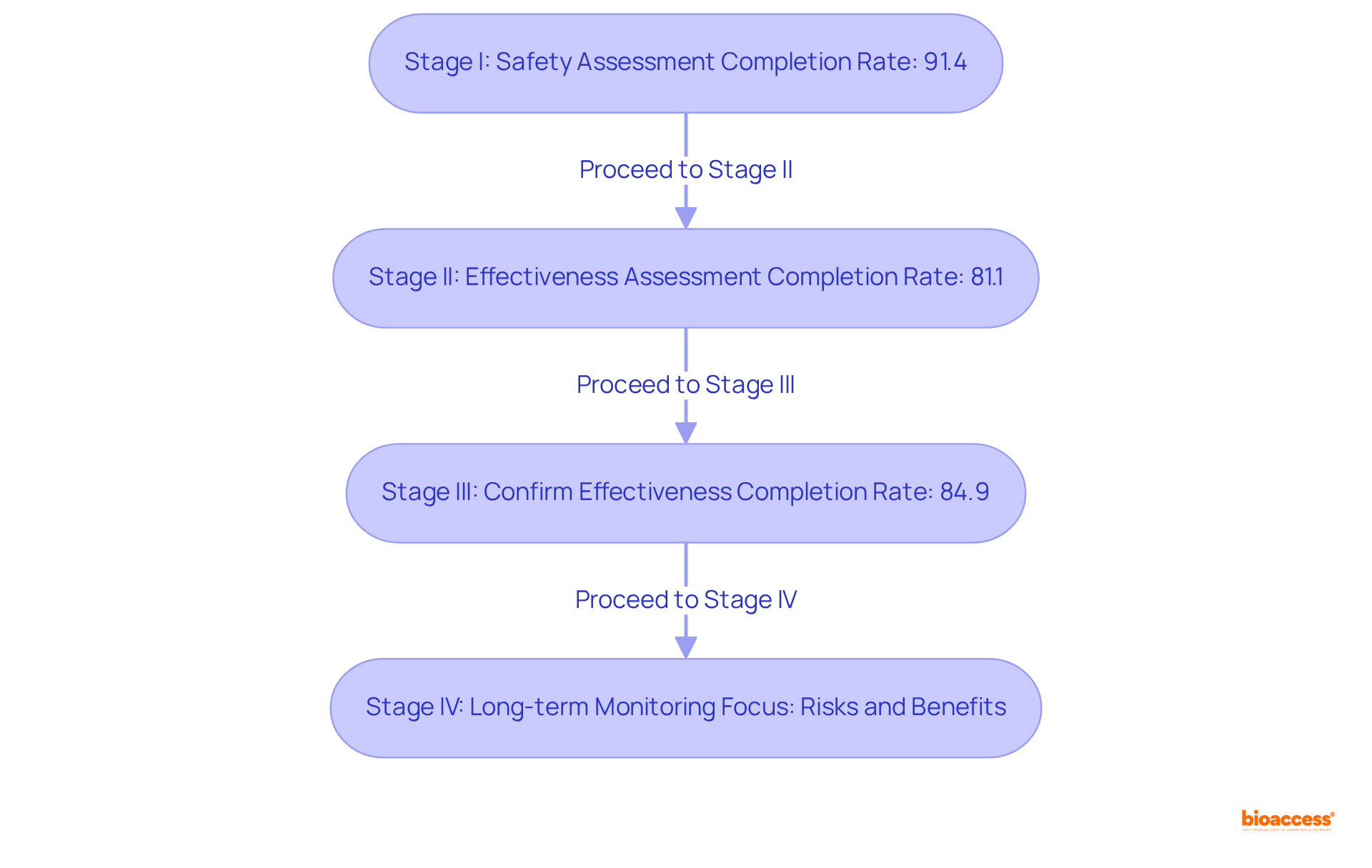
The clinical development phase is systematically organized into four distinct stages: Stage I, Stage II, Stage III, and Stage IV, each serving a pivotal role in the drug development process.
Stage I: This preliminary stage emphasizes safety, involving a small cohort of healthy volunteers, typically numbering between 15 to 50 participants. The primary objectives are to assess the drug's safety, establish a safe dosage range, and identify any side effects. Successful Stage I tests are essential for advancing to subsequent stages, with a notable completion rate of approximately 91.4%.
Stage II: In this stage, the medication is administered to a larger group, generally between 25 and 100 patients, to evaluate its effectiveness and further assess safety. This phase is crucial for determining whether the drug performs as intended, with a completion rate of around 81.1% for studies.
Stage III: This stage encompasses a significantly larger participant pool, typically ranging from 1,000 to 3,000 individuals. The focus here is on confirming the drug's effectiveness, monitoring side effects, and comparing it to existing treatments. Successful completion of Stage III is often a prerequisite for regulatory approval, with a fulfillment rate of 84.9% for studies.
Stage IV: Following regulatory approval, Stage IV studies are conducted to gather extensive information on the drug's long-term risks, benefits, and optimal use in the general population. This phase is vital for ongoing safety monitoring and can involve thousands of participants, contributing to a deeper understanding of the treatment's impact over time.
Current statistics reveal that the median duration for Phase I studies is approximately 1.6 years, while Phase II studies average around 2.9 years, and Phase III studies typically last about 3.8 years. Notably, oncology studies can have an average duration of 13.1 years, underscoring the extended timelines often associated with specific therapeutic areas. The overall probability of success (POS) for drug development programs has exhibited variability, with recent data indicating a POS of 6.2% for orphan drug development.
Understanding these stages is crucial for navigating the complexities of clinical studies and ensuring the effective management during the clinical development phase of new treatments. Additionally, bioaccess® facilitates ethical approvals in 4-6 weeks and secures enrollment 50% faster than traditional markets, highlighting the advantages of conducting clinical studies in the regions where it operates. Furthermore, informed consent and the oversight of Institutional Review Boards (IRBs) are essential components that uphold ethical standards throughout the clinical trial process.
To effectively navigate each phase of clinical development, it is essential to consider the following best practices:
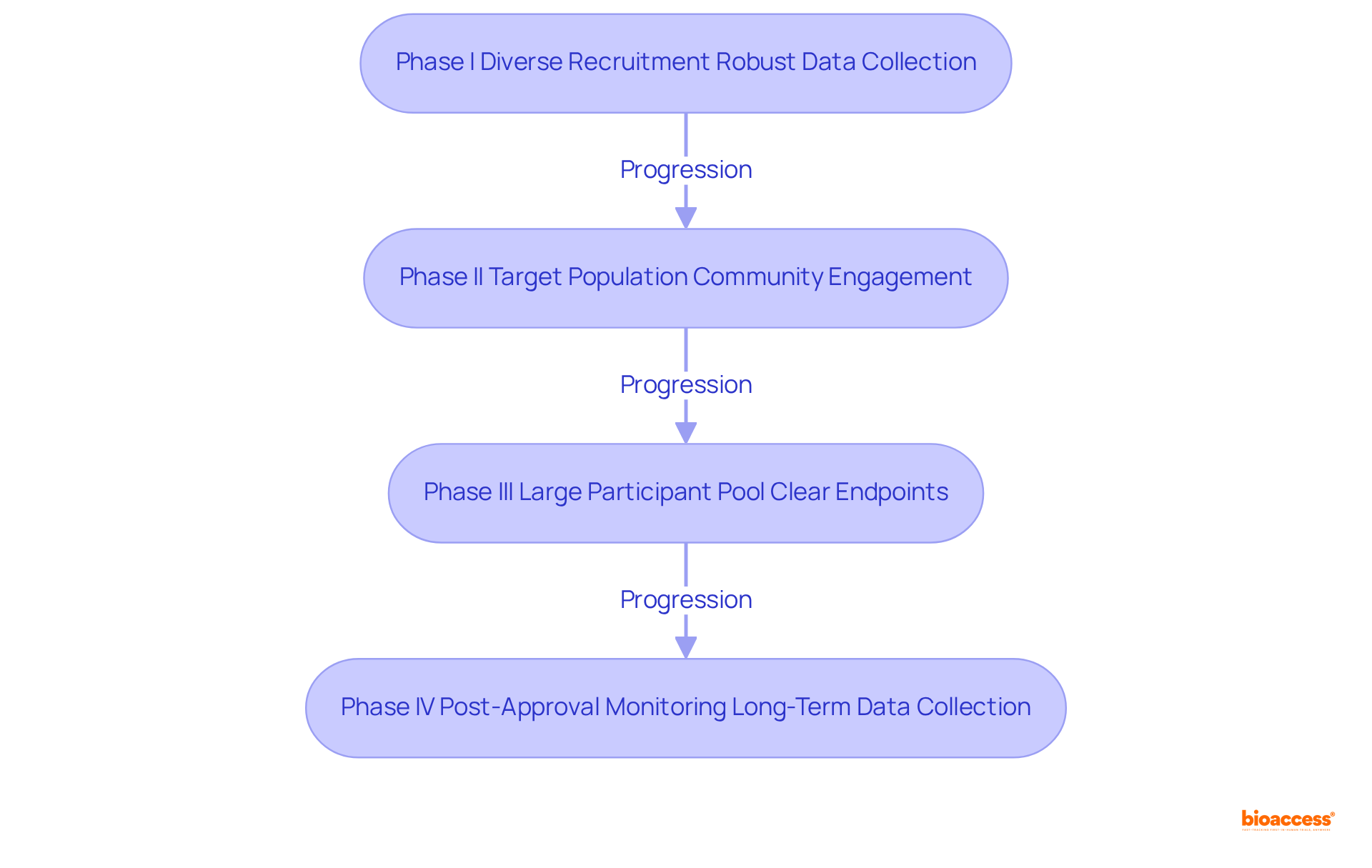
Phase I Navigation: Recruit a diverse group of healthy volunteers to ensure comprehensive safety data, as diversity correlates with improved generalizability of results. Notably, the average time to enroll a diverse cohort has increased to over 18 months due to recruitment barriers, particularly for underrepresented minorities. Close observation of individuals for any negative effects is crucial, allowing for dosage modifications as needed to ensure safety and effectiveness. It is advisable to employ specific strategies that have demonstrated improvement in results during the clinical development phase. Additionally, meticulous data collection and analysis are imperative to prepare for the clinical development phase, recognizing that thorough data collection is crucial for informed decision-making.
Phase II Navigation: Focus on recruiting participants who have the condition the drug is intended to treat, ensuring that the study accurately reflects the target population. Implementing robust data collection methods is essential to evaluate both efficacy and safety in the clinical development phase, preparing for potential adjustments in study design based on preliminary results. Furthermore, utilizing community engagement programs, which have shown to increase minority enrollment by up to 60%, can significantly enhance recruitment efforts. As noted by Alexander Eser, these programs are vital for building trust and improving participation rates.
Phase III Navigation: Ensure a large and diverse participant pool to enhance the validity of results, as geographic diversity in trial sites can lead to over a 50% increase in minority participation. Establishing clear endpoints for efficacy and safety during the clinical development phase is crucial to guide the analysis, while collaborating with regulatory bodies early on helps align expectations for data submission. It is important to acknowledge that logistical challenges are major obstacles to involvement, with around 70% of potential attendees residing over two hours from study centers, emphasizing the necessity for creative recruitment approaches.
Phase IV Navigation: Continue monitoring participants post-approval to gather long-term safety data, engaging with healthcare providers to understand real-world drug performance. Using findings to inform future research and product improvements ensures that the insights gained contribute to advancing healthcare innovations. Moreover, acknowledging that cultural mistrust and unmet enrollment goals hinder diverse representation emphasizes the need for trust-building and targeted outreach to achieve equitable healthcare innovation.
To ensure regulatory compliance and uphold ethical standards in clinical research, it is imperative to follow these essential steps:
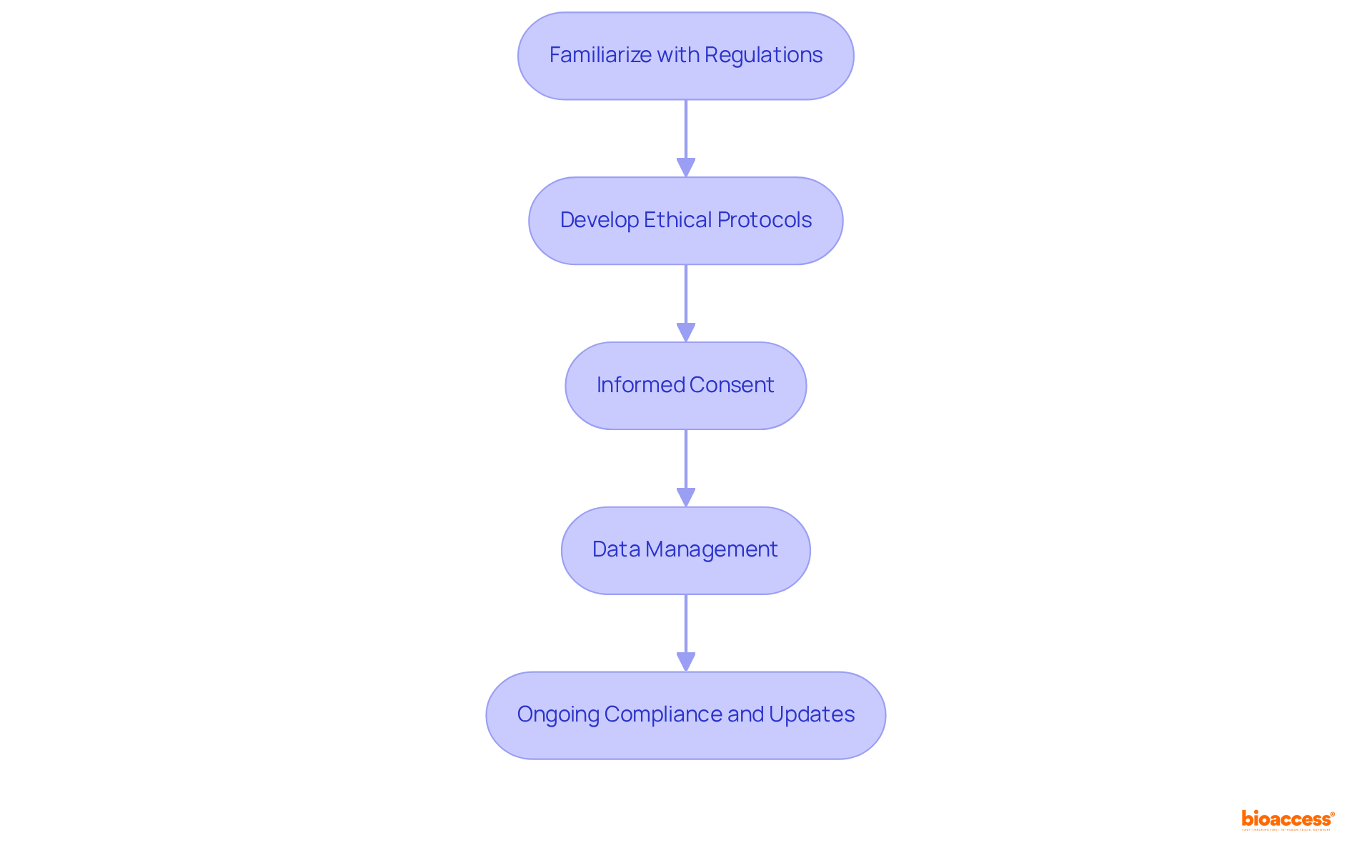
Familiarize with Regulations: Gain a thorough understanding of the regulations established by local and international bodies, such as the FDA and EMA. Staying informed about regulatory changes is crucial, as these can significantly impact your study's design and execution.
Develop Ethical Protocols: Craft a comprehensive study protocol that clearly outlines the objectives, methodology, and ethical considerations. This protocol must undergo review and approval by an Institutional Review Board (IRB) or Ethics Committee to ensure it meets ethical standards.
Informed Consent: Secure informed consent from all participants, ensuring they fully comprehend the study's purpose, potential risks, and benefits. Participants should feel empowered to ask questions and have the right to withdraw from the study at any time without facing penalties.
Data Management: Establish robust data management practices to safeguard participant confidentiality and maintain data integrity. Regular audits of data collection processes are essential to ensure adherence to ethical standards and regulatory compliance.
Recent updates highlight the critical nature of ethical adherence in clinical studies, with the FDA supporting Diversity Action Plans aimed at improving participant representation across various demographics. Statistics suggest that following ethical guidelines not only builds trust but also enhances study outcomes, with enrollment occurring 50% quicker than conventional markets. This underscores the necessity for sponsors and CROs to prioritize these standards. Successful ethical protocols have been shown to lead to more equitable healthcare advancements, emphasizing the importance of maintaining high ethical standards in clinical research. As Cetas Healthcare states, "Our results continually set the gold standard regarding regulatory compliance and MedTech market research." Additionally, the upcoming FDA guidance on single IRB reviews will streamline the ethical review process, further supporting the need for robust compliance measures.
To effectively manage recruitment and project timelines during the clinical development phase of clinical research, it is essential to implement strategic approaches that enhance efficiency and participation.
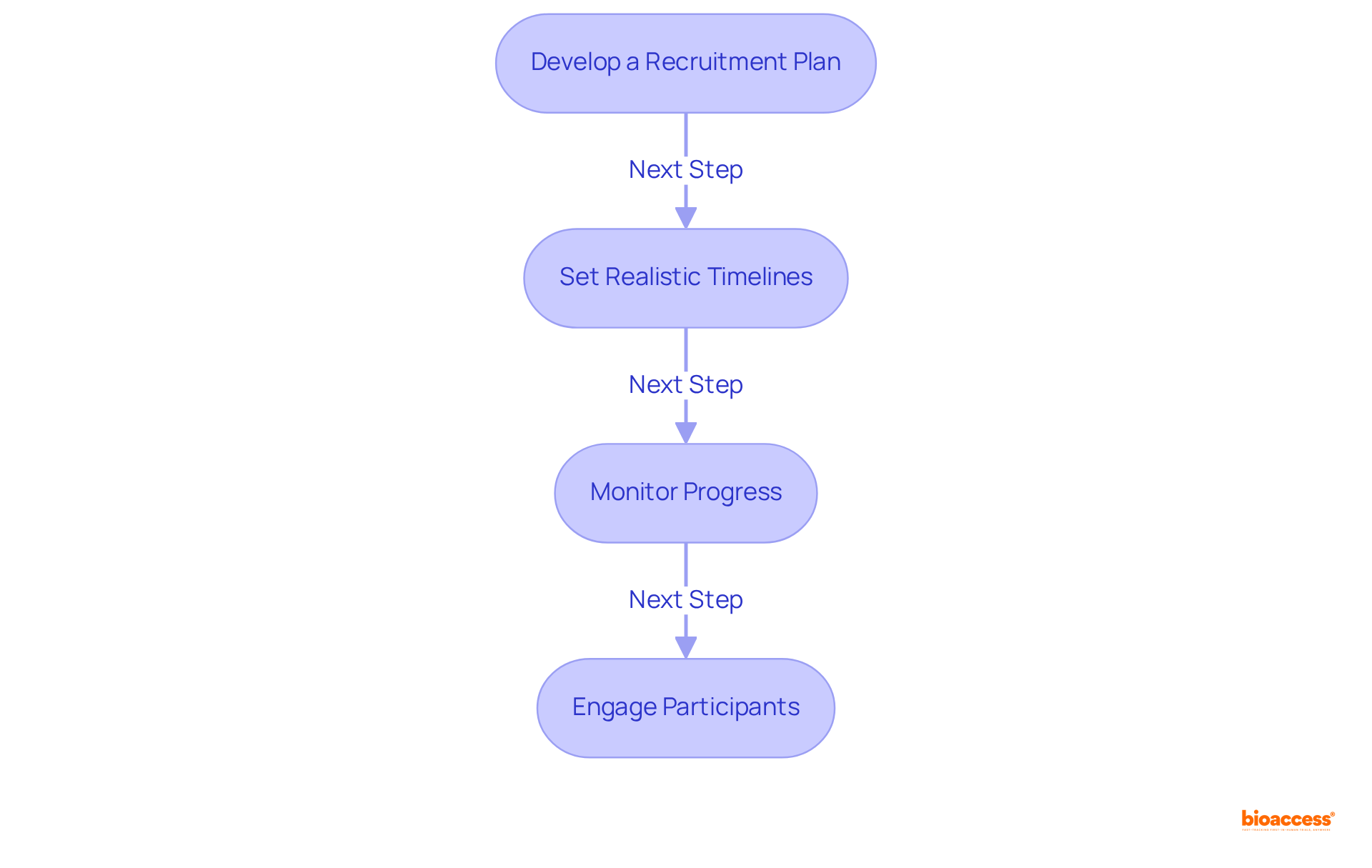
Develop a Recruitment Plan: Begin by identifying target populations and tailoring recruitment strategies to address their specific needs. Engaging various communities significantly boosts participation rates, as research shows that minorities often exhibit heightened interest in observational studies. Furthermore, leveraging diverse recruitment channels—such as social media, healthcare providers, and community outreach—maximizes visibility and outreach.
Set Realistic Timelines: Construct a comprehensive project timeline that encompasses all trial phases, from recruitment through to data analysis. Notably, the average recruitment duration for studies in the clinical development phase, specifically phase III clinical studies, has increased from 13 months to 18 months over the past decade, underscoring the critical need for meticulous planning to avert costly delays. Employing project management tools, like Gantt charts, facilitates the visualization of timelines and dependencies, ensuring that all team members remain aligned on expectations.
Monitor Progress: Regular assessment of recruitment metrics is vital to identify any shortfalls and adapt strategies as necessary. Approximately 80% of clinical studies face delays or shutdowns in the clinical development phase due to recruitment challenges, which can result in costs ranging from $600,000 to $8 million for each day a study is postponed, highlighting the importance of proactive oversight. Conducting weekly or bi-weekly meetings with the research team allows for discussions on progress, addressing challenges, and recalibrating efforts as needed.
Engage Participants: Maintaining open communication with participants throughout the trial is crucial for improving retention rates. Research indicates that 73% of patients prefer to learn about clinical trial opportunities from their healthcare providers, emphasizing the necessity of nurturing strong relationships. Providing regular updates on the study's progress and any protocol changes ensures that participants feel informed and valued throughout their involvement.
Mastering the clinical development phase is essential for the successful approval and implementation of new drugs. This systematic process, divided into four key stages—Stage I, Stage II, Stage III, and Stage IV—ensures that safety, efficacy, and ethical standards are rigorously upheld. Each stage serves a unique purpose, from initial safety assessments with healthy volunteers to comprehensive evaluations involving thousands of patients, culminating in long-term monitoring post-approval. Understanding these phases is critical for navigating the complexities of clinical trials and ensuring that new treatments are both effective and safe for public use.
Throughout the article, key insights have been provided on how to effectively manage each phase of clinical development. The importance of diverse participant recruitment, robust data collection, and adherence to ethical standards has been emphasized. Strategies for enhancing recruitment, setting realistic timelines, and maintaining open communication with participants are vital for overcoming common challenges faced in clinical trials. Additionally, the role of regulatory compliance and ethical protocols in fostering trust and improving study outcomes has been highlighted, showcasing their significance in the drug development process.
In conclusion, the clinical development phase is not merely a procedural formality; it is a foundational aspect of healthcare innovation that requires diligence and strategic planning. As the landscape of clinical trials continues to evolve, stakeholders must prioritize effective management practices and ethical considerations to ensure equitable access and representation in research. By embracing these principles, the potential for groundbreaking advancements in medicine can be realized, ultimately benefiting patients and communities worldwide.
What are the main stages of clinical development?
The clinical development phase is organized into four stages: Stage I, Stage II, Stage III, and Stage IV, each serving a crucial role in the drug development process.
What is the focus of Stage I in clinical development?
Stage I emphasizes safety and involves a small group of healthy volunteers (15 to 50 participants). Its primary objectives are to assess the drug's safety, establish a safe dosage range, and identify any side effects. The completion rate for Stage I tests is approximately 91.4%.
How does Stage II differ from Stage I?
Stage II involves a larger group of patients (25 to 100 individuals) to evaluate the drug's effectiveness and further assess safety. This stage is crucial for determining whether the drug performs as intended, with a completion rate of around 81.1%.
What is the purpose of Stage III in clinical trials?
Stage III includes a significantly larger participant pool (1,000 to 3,000 individuals) and focuses on confirming the drug's effectiveness, monitoring side effects, and comparing it to existing treatments. Successful completion of Stage III is often required for regulatory approval, with a fulfillment rate of 84.9%.
What occurs during Stage IV of clinical development?
Stage IV studies are conducted after regulatory approval to gather extensive information on the drug's long-term risks, benefits, and optimal use in the general population. This phase is vital for ongoing safety monitoring and can involve thousands of participants.
How long do the different stages of clinical development typically take?
The median duration for Phase I studies is approximately 1.6 years, Phase II studies average around 2.9 years, and Phase III studies typically last about 3.8 years. Oncology studies can have an average duration of 13.1 years.
What is the probability of success (POS) for drug development?
The overall probability of success for drug development programs has varied, with recent data indicating a POS of 6.2% for orphan drug development.
What role do ethical approvals play in clinical trials?
Ethical approvals are essential for upholding ethical standards throughout the clinical trial process. Informed consent and oversight by Institutional Review Boards (IRBs) are critical components of this process.
How does bioaccess® improve the clinical study process?
Bioaccess® facilitates ethical approvals in 4-6 weeks and secures enrollment 50% faster than traditional markets, highlighting the advantages of conducting clinical studies in the regions where it operates.